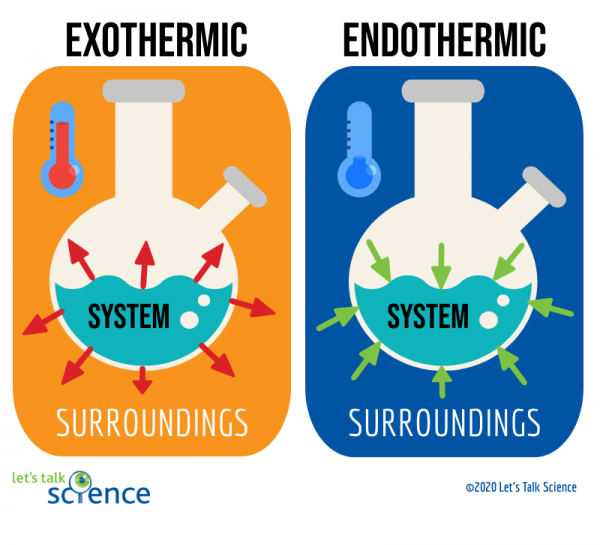
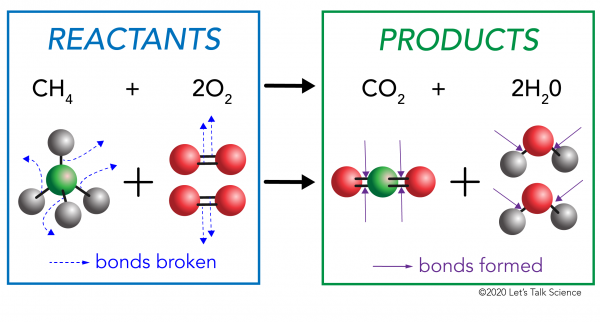
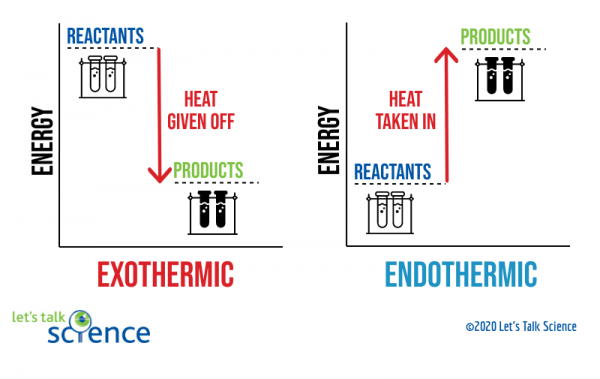
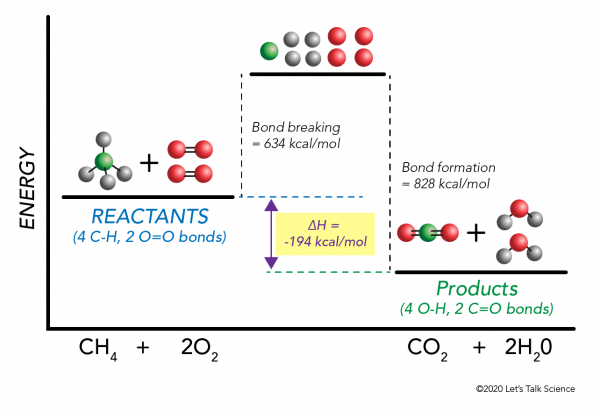
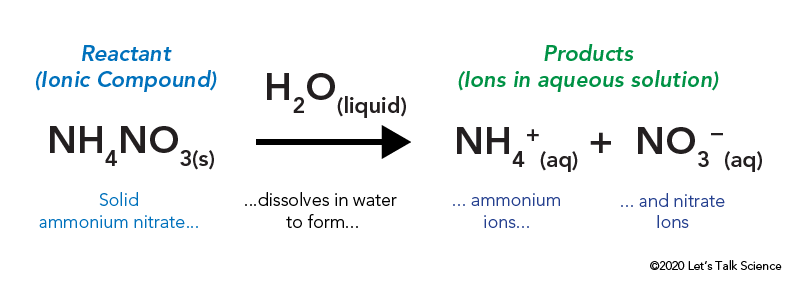
The Cold Pack: A Chilly Example of an Endothermic Reaction
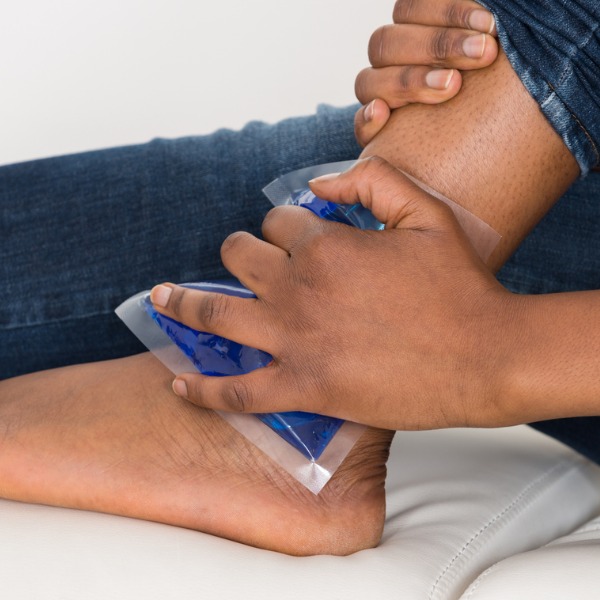
A person’s hand holding a cold pack on an ankle (AndreyPopov, iStockPhoto)

A person’s hand holding a cold pack on an ankle (AndreyPopov, iStockPhoto)
8.69
How does this align with my curriculum?
Curriculum Alignment