Introduction to Quantum Mechanics

Waves and particles (agsandrew, iStockphoto)

Waves and particles (agsandrew, iStockphoto)
How does this align with my curriculum?
| Grade | Course | Topic |
|---|
Learn what quantum mechanics is, the history of its development and the ways we use it today.
Quantum mechanics may sound intimidating. But it can be a lot of fun to learn about! It really makes you ‘stretch’ your brain and expand your critical thinking skills. And quantum mechanics doesn’t just happen in high-tech physics labs. The applications of quantum mechanics are all around us. Even inside the device you’re using to read this article!
So what is quantum mechanics anyway?
Essentially, quantum mechanics is the study of how atomic particles exist and interact with each other.
You might be familiar with classical mechanics, such as Newton’s three laws of motion. Classical mechanics allows scientists to make very accurate predictions for big objects. But these predictions do not work as well when you look at objects on a smaller scale.
This is where quantum mechanics comes in. It describes laws of energy on the scale of atoms. The best way to understand quantum mechanics is through the history of its major discoveries.
A Brief History of Quantum Mechanics
1900: Planck and Quanta
At the turn of the twentieth century, many physicists thought there was nothing left to discover in their field. However, one big problem remained. This had to do with hot objects.
Think of your toaster or your stove top. When the elements get hot, they glow red. If you were able to increase the temperature even more, they’d glow white. This pattern of colour change with temperature is the same for any object, no matter what the object is made out of.
At first, scientists used classical physics to try to understand this pattern. Their models predicted that hot objects should emit light that is mostly in the ultraviolet frequency range.
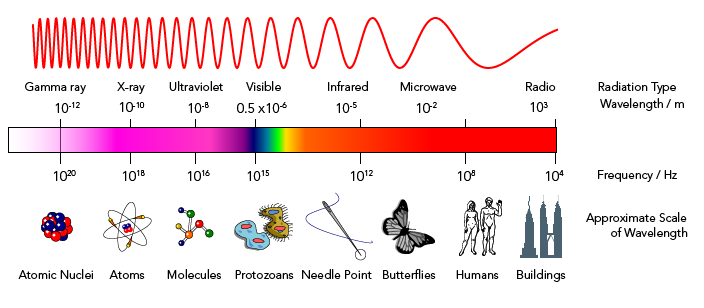
But their experiments showed that almost no ultraviolet light was emitted at all! This problem was called the ultraviolet catastrophe. It was solved by Max Planck, a German theoretical physicist. He is often called the father of quantum mechanics.
Planck created a new mathematical formula for the frequencies of light energy emitted from a hot object. It showed that warm objects would emit red frequencies. Hotter objects would emit frequencies of all visible colours, making them appear to glow white. Most importantly, Planck’s formula predicted that no ultraviolet frequencies would be emitted. It fit the experimental evidence perfectly!
Planck’s formula worked because of one key idea. Before Planck, scientists believed that energy was on a continuous scale. They thought that it was possible for an object to have any energy value on that scale. Planck’s radical hypothesis was that at the sub-atomic level, hot objects could only emit energy in small units or “packets.” He called these packets quanta (a single quanta is called a quantum). Planck said the amount of energy in a quantum increased with its frequency. Lower frequencies, like red light, have less energy than higher frequencies, like those in the white light.
However, Planck didn’t have a reason for why energy was quantized in this way. He wrote in a letter to a colleague that making this mathematical assumption in his formula had been an “act of desperation.” The answer to this would come from Niels Bohr, 13 years later.
1905: Einstein and Photons
But before Bohr, quantum theory helped solve another problem in the physics world - the photoelectric effect. This is the observation that shining light onto a metal surface can cause electrons to eject from the metal.
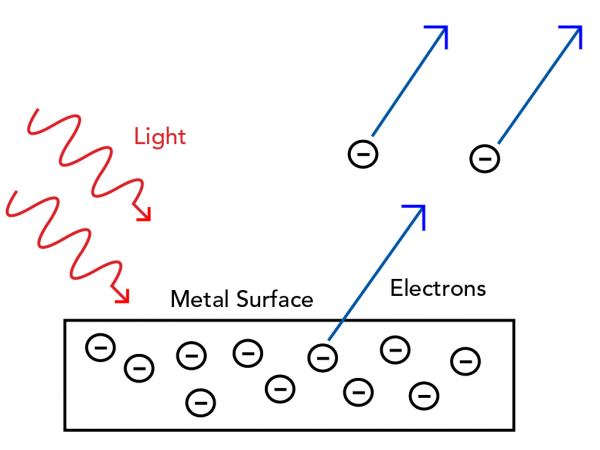
Classical mechanics describes light as a wave. The height of a wave is called its amplitude. You would expect a wave with a greater amplitude to push electrons off the surface with more energy. The time between wave peaks is called its frequency. You would expect waves with higher frequency to hit and eject more electrons off the surface.
But as with most quantum experiments, the unexpected was observed. When metal was exposed to light, a bigger amplitude caused more electrons to eject. And light with a higher frequency caused electrons to eject with more energy. Classical physics could not explain this!
|
Classical Predictions |
Quantum Observations |
||
|
Greater amplitude |
Greater energy |
Greater amplitude |
More electrons eject |
|
Higher frequency |
More electrons eject |
Higher frequency |
Greater energy |
But what could explain it? Albert Einstein, the famous German physicist, had a theory. He applied Planck’s quantum explanation to light. He theorized that light sometimes behaves like discrete packets of electromagnetic energy. He called these packets photons.
Did you know?
Einstein received his Nobel Prize in Physics in 1921 not directly for his theory of relativity, but for “his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect.”
If a beam of light has a bigger amplitude, this means it contains more photons. If more photons strike the metal surface, there are more collisions, so more electrons are ejected.
The energy contained in one photon depends only on its frequency. This is just like Planck’s quanta of energy. So, light with a higher frequency will transfer more energy to the electrons, which is what was observed experimentally.
1913: Bohr and Electron Orbitals
Quantum theory was gaining traction. But it was still just a mathematical explanation for some strange observations. No one could explain why energy came in discrete packets. Until Neils Bohr, that is. In 1913, the Danish physicist proposed a new model for the structure of the atom.
Before Bohr, scientists knew that an atom is made of a positively charged nucleus with negatively charged electrons orbiting around it. Bohr revolutionized this model. He said that those electrons had to lie on one of a set of specific paths. These paths were like planets' orbits around the Sun. He called them electron orbitals. Each orbital has an associated energy level.
When an electron absorbs enough energy, it “jumps” from one orbital to the next biggest orbital. When an electron “falls” from one orbital to the next smallest it emits energy. The amount of energy emitted is exactly the energy difference between the two orbitals. This is why energy exists in discrete values, as “quanta,” rather than on a continuous scale.
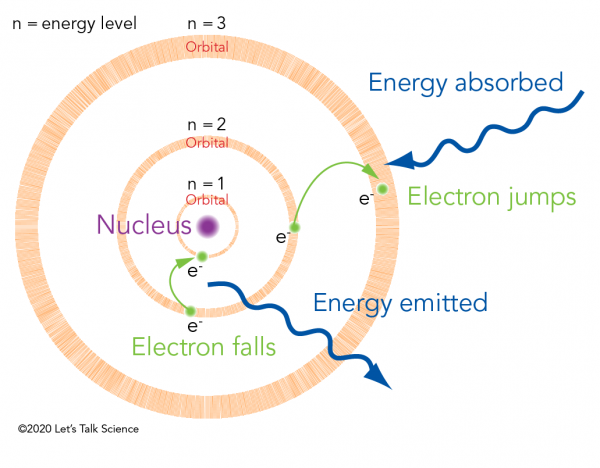
The quantized energy that Planck saw was electromagnetic radiation emitting from electrons in the hot objects. Einstein’s photons, on the other hand, transferred their energy to electrons in the metal. If the photon energy was high enough, the electron would leave its orbital and leave the metal altogether. So, Bohr’s electron orbitals provided a theoretical explanation for quantum mechanics.
Quantum Mechanics Today
Planck, Einstein, Bohr (and many others) won the Nobel Prize in Physics for their work in quantum mechanics. But if they were alive today, the world would be unrecognizable to them, because of their own discoveries!
Did you know?
We have quantum theory to thank for Magnetic Resonance Imaging (MRI). This helps doctors take pictures of the inside of your body.
Computers run on quantum mechanics. But why? Because of transistors.
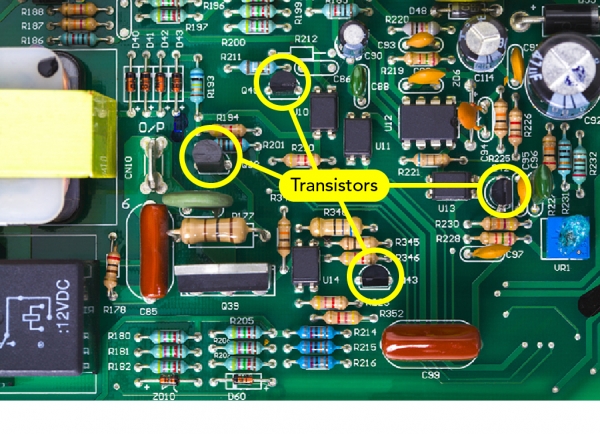
Transistors are tiny electronic pieces inside of computers that store bits of information. When a transistor is turned on, it conducts electricity, and the computer reads this as a “1.” When a transistor is turned off, it no longer conducts electricity, and the computer reads this as a “0.” Computers speak in a language of 1s and 0s. They turn transistors on or off to encode information.
What does this have to do with quantum mechanics? Well, transistors are made out of a material that is a semiconductor.
Quantum mechanics tells us that an electron can only occupy certain energy levels. When looking at a large group of electrons, like those found in semiconductors, these levels are “bands,” or ranges of allowed energy values.
When the semiconductor is connected to a voltage that is inside of the energy band, it conducts electricity. When it is connected to a voltage outside of the allowed energy band, it does not conduct electricity. It acts as an insulator. This is how transistors turn on or off, which the computer reads as 1 or 0.
Did you know?
Silicon is the most common semiconducting material used in computer transistors. This is how the famous Silicon Valley got its name. This part of California is considered a major centre of technological innovation.
Something that uses a binary code of 1s and 0s is called digital. Almost any digital device you can think of uses semiconducting transistors. Your computer, your cell phone, even your television! Imagine the world without these things, or without the Internet. This would be the world without quantum mechanics.
Starting Points
- What do you know about quantum mechanics?
- Can you think of any devices/equipment/appliances that you use that might require transistors to operate?
- How has the field of quantum mechanics impacted technology?
- Max Planck and Albert Einstein were theoretical physicists. Why is the study of theoretical physics important?
- What is the difference between classical mechanics and quantum mechanics?
- Where did the name quantum mechanics come from?
- How did the work of Neils Bohr connect to the previous theories of Planck and Einstein?
- What is a transistor and how does it encode information?
- How does the history of quantum physics outlined in this article demonstrate that science is a dynamic process?
- Have you watched any movies about Max Planck, Albert Einstein or Neils Bohr? Which scientist has a greater presence in popular media? What could be the reason(s) for this?
- This article supports teaching and learning of Physics related to modern physics and quantum physics. Concepts introduced include quanta, quantum, photoelectric effect, amplitude, frequency, Albert Einstein, Neils Bohr and semiconductors.
- To help consolidate information from the article, students could use a (Five W’s and an H)² learning strategy. Ready-to-use reproducibles for this article are available in [Google doc] and [PDF] formats.
After reading this article, teachers could have students use a BYO (Building Your Own) Timeline learning strategy to outline the early developments of quantum mechanics presented in the article. Ready-to-use BYO (Building Your Own) Timeline reproducibles for this article are available in [Google doc] and [PDF] formats.
Connecting and Relating
- What do you know about quantum mechanics?
- Can you think of any devices/equipment/appliances that you use that might require transistors to operate?
Relating Science and Technology to Society and the Environment
- How has the field of quantum mechanics impacted technology?
- Max Planck and Albert Einstein were theoretical physicists. Why is the study of theoretical physics important?
Exploring Concepts
- What is the difference between classical mechanics and quantum mechanics?
- Where did the name quantum mechanics come from?
- How did the work of Neils Bohr connect to the previous theories of Planck and Einstein?
- What is a transistor and how does it encode information?
Nature of Science/Nature of Technology
- How does the history of quantum physics outlined in this article demonstrate that science is a dynamic process?
Media Literacy
- Have you watched any movies about Max Planck, Albert Einstein or Neils Bohr? Which scientist has a greater presence in popular media? What could be the reason(s) for this?
Teaching Suggestions
- This article supports teaching and learning of Physics related to modern physics and quantum physics. Concepts introduced include quanta, quantum, photoelectric effect, amplitude, frequency, Albert Einstein, Neils Bohr and semiconductors.
- To help consolidate information from the article, students could use a (Five W’s and an H)² learning strategy. Ready-to-use reproducibles for this article are available in [Google doc] and [PDF] formats.
After reading this article, teachers could have students use a BYO (Building Your Own) Timeline learning strategy to outline the early developments of quantum mechanics presented in the article. Ready-to-use BYO (Building Your Own) Timeline reproducibles for this article are available in [Google doc] and [PDF] formats.
Learn more
De Broglie wavelength (2017)
In 1924, Louis De Broglie said that just like waves can have the properties of particles, particles can have the properties of waves. This video from Khan Academy explains (11:19 min.).
No discussion of quantum mechanics is complete without Schrödinger’s cat! This IFLScience article with video (7:57 min.) explains how Schrodinger developed the paradox of the cat inside of a box who is both dead and alive at the same time.
The Ultraviolet Catastrophe (2016)
In this video (6:31 min.), Physics Girl illustrates the historical importance of the Ultraviolet Catastrophe to the development of quantum mechanics.
References
Biercuk, M. (2011, May 15). Explainer: Quantum physics. The Conversation.
The Editors of the Encyclopedia Britannica. (2016). De Broglie wave.
The Editors of Encyclopedia Britannica. (2019). Semiconductor.
The Editors of Encyclopedia Britannica. (2020). Bohr model.
George, A., A. (2014, February 11). Classical mechanics vs quantum mechanics. ClearIAS.
Helmenstine, A. M. (2020, January 27). Bohr model of the atom - Overview and examples. ThoughtCo.
High School Physics Explained. (2016, June 18). Black bodies and Planck explained.
IFLScience. (n.d.). Schrödinger's cat: Explained.
Khan Academy. (n.d.). Photoelectric effect (article).
Matson, J. (2010, November 2). What is quantum mechanics good for? Scientific American.
The Nobel Prize. (2020). All nobel prizes in physics.
Orzel, C. (2018, December 4). Three ways quantum physics affects your daily life. Forbes.