Introduction to Static Electricity
Getting a shock of static electricity (ericb007, iStockphoto)

Getting a shock of static electricity (ericb007, iStockphoto)
How does this align with my curriculum?
Learn about static electricity and where we encounter it in daily life.
Static Electricity
You’ve probably felt static electricity. It causes that little shock you can get when you first touch a doorknob. It can also make your hair stand on end when you pull off your toque in the winter. But do you know why these things happen?
All matter is made up of atoms or groups of atoms called molecules. In the center of each atom is the nucleus. Inside the nucleus, there are particles with positive charges (+) and particles with a charge of zero. The ones with positive charges are called protons and the ones with a charge of zero are called neutrons. Particles with negative charges (-), called electrons, orbit around the nucleus.
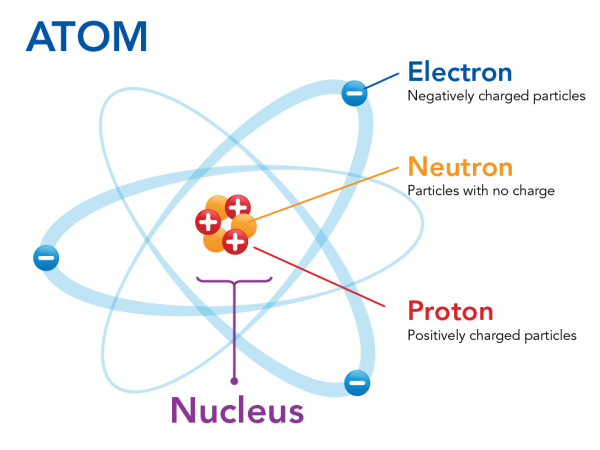
Normally, the objects and materials around us are electrically neutral. This means that they don’t have either a positive or a negative charge. This is because they have an equal number of positive charges from the protons and negative charges from the electrons.
Did you know?
The study of electric charges at rest is called electrostatics.
But it is possible to give a neutral material an electric charge. One way to do this is by rubbing two objects together.
When you go down a slide, electrons move from you to the slide. This causes your hairs to have more positive charges. Objects that have the same charge repel each other. So, each of your hairs tries to repel its neighbour. Sometimes this means your hair will stand straight up!
The same thing happens when you rub a balloon against your hair or clothes, especially in dry weather. Try putting your balloon on the wall afterwards and see how long it sticks! By rubbing, you move electrons from you to the balloon. It sticks because the balloon is more negatively charged than the wall. Objects with opposite charges are attracted to each other.

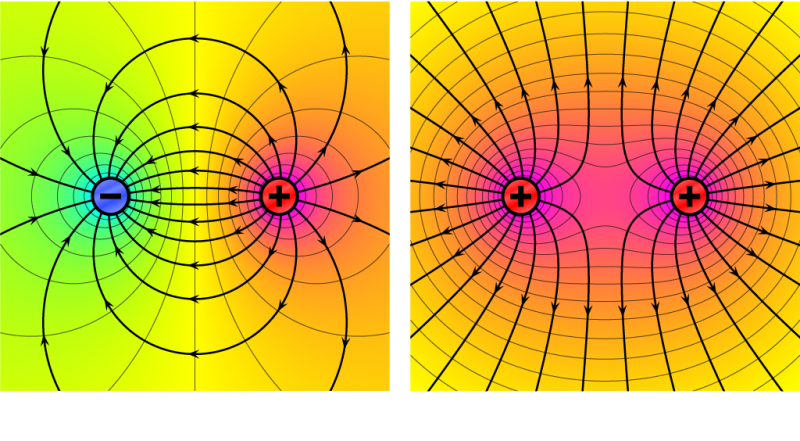
But why do charged particles repel or attract each other? Because they can exert a force around themselves. This is called an electric force. The area around the particle affected by this force is called an electric field. We can show what an electric field looks like by drawing pictures with arrows called field lines. You can see examples in the pictures below.
Electric field lines always point away from positive charges. But they point towards negative charges. If particles have opposite charges, their field lines point towards each other. If particles have the same charge, their field lines point away from each other.
Did you know?
Particle charges travel better when there is moisture in the air. This means objects don’t have a chance to build up positive or negative charges. But the opposite can happen on a dry winter day. That’s why your hair might look funny when you take off your toque!
If two materials with opposite charges get close to each other, they may create a spark. This spark is the movement of electrons through the air! You may have felt this when reaching for a doorknob after walking along the carpet.
We also see this same kind of zap of electricity during a thunderstorm. We call these zaps lightning.

During a storm, clouds pass by each other. Electrons can jump from one cloud to another. Because of this, some of the clouds can develop large positive or negative electrical charges. These opposite charges are strongly attracted to each other. Eventually they ‘jump’ through the air to equalize themselves. The result is a spectacular flash of light.
Lightning can happen inside a cloud, between clouds and between clouds and the ground. Lightning is the most powerful form of static electricity you can experience. This is why thunderstorms can be very dangerous. Remember, when thunder roars, go indoors.
Electrostatic Force
The zap of lightning is a lot stronger than the zap you might feel when you touch a doorknob! But why is that?
We know that positive and negative charges interact with each other. But the strength of this interaction is measured by the size of the electrostatic force. This force is caused by both the size of the electric charges and the distance between the charges. Let’s look at this using a diagram.
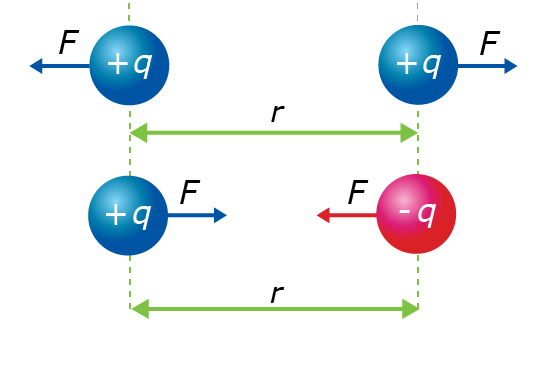
The particles with a positive charge are labelled as +q and particles with a negative charge as -q. The distance between the particles is labelled as r. The forces acting on the charges are labelled as F.
You can calculate the electrostatic force between two particles using Coulomb’s Law. This equation describes the relationship between the charges of the particles and the distance between the particles. The symbol k represents the Coulomb's Law constant.
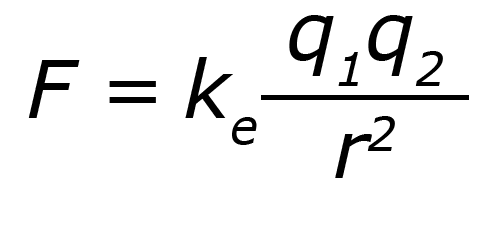
There are two important things to remember when looking at this equation.
- The values of q1 and q2 are multiplied together. So, if both q1 and q2 are positive, then the force will be a positive value. The particles will repel.
The same is true if both q1 and q2 are negative.
If q1 is positive and q2 is negative, then the force will be a negative value. The same is true if q1 is negative and q2 is positive. The particles will attract.
- The force is proportional to the square root of the distance between the particles. This means that the closer the particles are to each other, the stronger the force will be.
Did you know?
Coulomb’s Law is named after physicist Charles-Augustin de Coulomb. He proved his law using an instrument called a torsion balance. This measured the static electrical force when two balls of charged metal repelled or attracted each other. The coulomb, a unit of electric charge, was named in his honour.
Learn More
How can I move water with just a comb?
This hands-on activity shows students how static electricity can affect a stream of water.
Thundersnow: winter thunderstorms
This Let's Talk Science article explains how thunder, lightning and precipitation work together to create storms, including the rare thundersnow.
Bill Nye the Science Guy Performs a Static Electricity Science Demonstration
This video by SophiaLearning demonstrates how static electrical charges attract and repel each other.
References
Rader’s Physics for Kids. (n. d.) Coulomb's Law.
The Physics Classroom. (n. d.) Physics Tutorial: Lightning
The Physics Classroom. (n. d.) Physics Tutorial: Static Electricity.
Wissner-Gross, J. (2011 December 28). Coulomb's law.