Osmosis and Its Role in Human Biology and Health
Human kidney cross-section (Mohammed Haneefa Nizamudeen, iStockphoto)

Human kidney cross-section (Mohammed Haneefa Nizamudeen, iStockphoto)
How does this align with my curriculum?
| Grade | Course | Topic |
|---|
Learn how and where osmosis takes place in the digestive system and excretory system and the role of osmosis in kidney dialysis.
Introduction
Imagine playing basketball with your friends on a hot summer day. By the end of the game, you feel thirsty. You decide to drink some water. But have you ever wondered how your body absorbs it?
It happens because of osmosis. We will look at how osmosis happens and why it is important for our bodies.
What is a semipermeable membrane?
Before we jump into osmosis, we need to understand some important things about cells. The cells in our bodies are surrounded by a wall-like structure called a cell membrane. This membrane is special because only water and very small molecules can pass through it. We use the word semipermeable to describe the ability to only let certain things pass through a membrane.
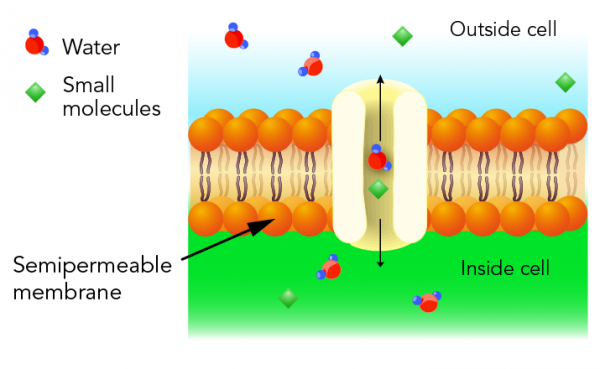
Why is this membrane important? It’s because water must pass through semipermeable membranes to travel from one place in our body to another.
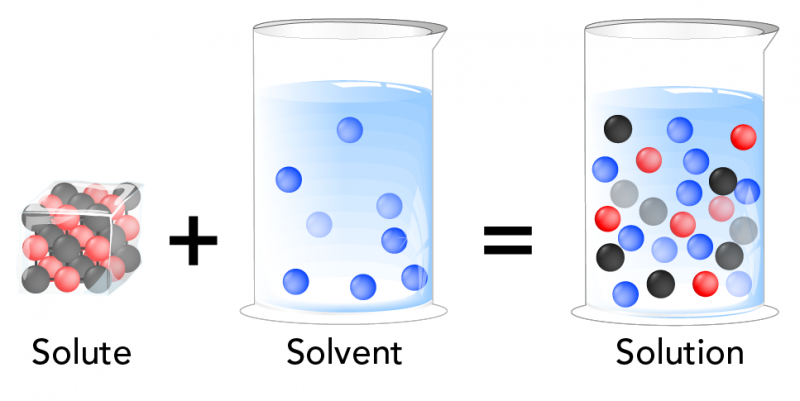
What are solutes and solvents?
Osmosis is when water molecules travel from a place with low solute concentration to a place with high solute concentration. To understand this better, we need to talk about solutes and solvents. A solute is a chemical that can dissolve in a solvent. Chemicals that can do this are called soluble. When you dissolve one or more solutes into a solvent, you get a solution. Sugar and salt are both chemicals that are soluble in water.
Did you know?
Water is often called “the universal solvent” because so many things can dissolve in it.
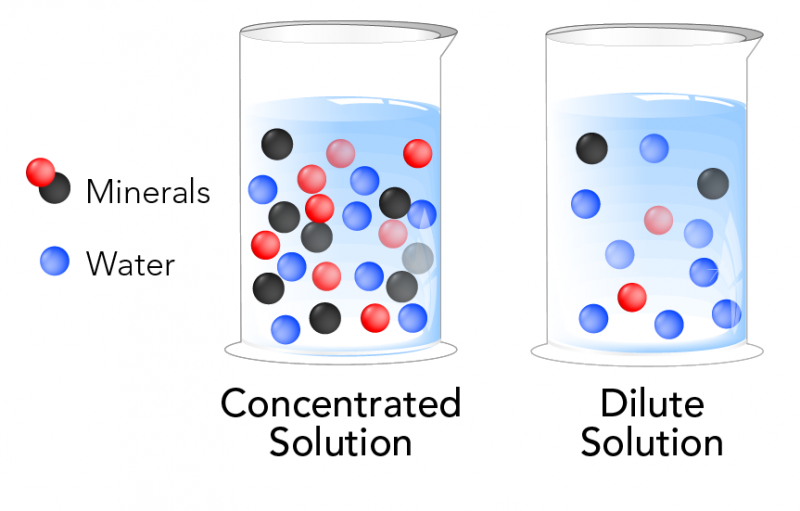
What is concentration?
You may have heard some products described as concentrated - like laundry detergent or orange juice. Concentrated refers to the amount of solute compared to the amount of solvent in a solution. When there is a lot of solute compared to solvent, a solution is said to be concentrated. When there is a small amount of solute compared to solvent, then a solution is said to be dilute.
Did you know?
Tap water is actually a solution! This is because tap water is not just H2O (water). It also contains minerals like calcium. In tap water, water is the solvent and the minerals are the solutes.
Did you know?
Osmolarity refers to the total concentration of all solutes in the solution.
When solutions of different osmolarities are separated by a membrane that lets water but not solutes pass through, water will move from the side with lower osmolarity (high concentration of water) to the side with higher osmolarity (lower concentration of water).
Moving water from place to place is what allows plants and animals to keep their levels of water and of nutrients in balance or equilibrium. In living things, all processes involved in maintaining conditions necessary for survival is called homeostasis. It’s kind of like the story of Goldilocks and the Three Bears. Organisms like to keep everything not too hot, not too cold, but just right!
Osmosis and our Gastro-intestinal System
So now that you know more about osmosis, it’s time to talk about your gastro-intestinal (GI) system - the parts of your body that deals with food and drink.
When you eat food or drink water, it travels from your mouth, down your esophagus and into your stomach. In the stomach, the food is broken into tiny pieces that are mixed with stomach liquids. This mush of food and stomach liquids is called chyme. The chyme travels into the small intestine. This is where osmosis takes place.
The chyme has a higher concentration than the epithelial cells that line your intestines. So, in order to reach homeostasis, water moves into these cells through their semipermeable membranes, taking small nutrients along with it. Near the epithelial cells are capillaries. The water and nutrients move through the cells of the capillaries and into the bloodstream.
Did you know?
About nine litres of fluid goes into your GI system each day and the small intestine absorbs 8 of those litres!
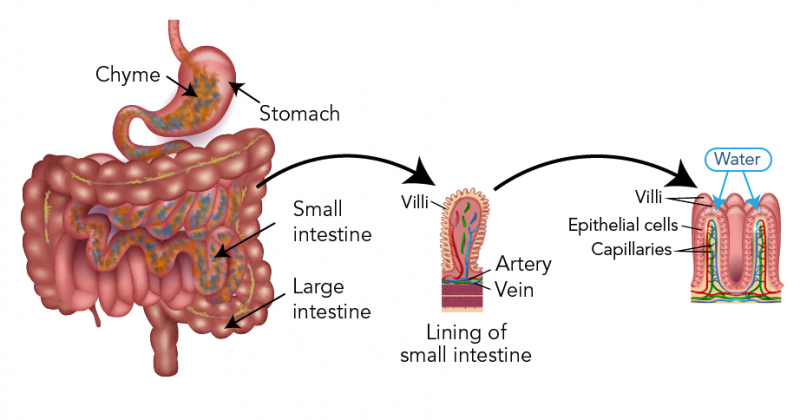
Graphic - Text Version
Chyme passes from the stomach into the small intestines. Within the small intestine are folds of tissue called villi. Water passes through the epithelial cells on the villi and into capillaries which carry blood.
Osmosis and our Kidneys
The water in your blood then travels to your kidneys. Kidneys are some of the most complex parts of the body, and they use osmosis as well.
Kidneys are made up of two parts - the cortex and medulla. The cortex is the outer part and the medulla is the inner part of the kidney.
The kidneys are made up of groups of cells called renal pyramids. Each pyramid contains little units called nephrons. Nephrons look like a bunch of tubes connected to each other. Nephrons are important because they help filter waste out of your blood and put it into your urine.
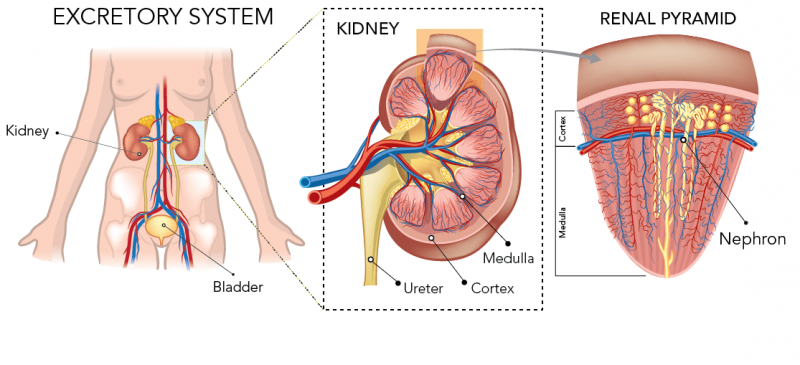
The largest part of each nephron is inside the medulla. The environment of the medulla has a higher osmolarity than the inside of the nephron. You know what that means - osmosis time! Water travels from inside the nephron tubes, through a semipermeable membrane, out into the medulla. Eventually, concentrated urine is left in the nephron. The urine travels through the ureter to the bladder.
Dialysis
So you can see that the kidneys have a vital role in your body. But what happens if one of your kidneys can’t do its job anymore? This is when doctors have to use dialysis to help.
A dialysis machine also uses a semipermeable membrane. It works in a similar way to a nephron. Blood is pumped next to a membrane that has dialysis fluid on the other side. Because of osmosis, the water in the blood, and very small molecules of waste, move across the membrane into the dialysis fluid. Eventually the dialysis fluid will remove all of the waste materials it can from the blood. That’s why dialysis can be life-saving for people who don’t have healthy kidneys.
Does osmosis cause your fingers to wrinkle in water?
Have you ever noticed how your fingers become wrinkly after sitting in the bath too long? You might think this is because osmosis is causing water to leave your finger cells and into the bath water. That’s what doctors used to think.
In the 1930s, Dr. T. Lewis and Dr. G.W. Pickering noted that people with nerve damage in their fingers did not experience this wrinkling. If your fingers only wrinkled because of osmosis, then nerve damage would not change it at all! Thus, it was discovered that osmosis is not responsible for the wrinkling, but it’s actually due to our sympathetic nerves.
Sympathetic nerves are a special type of nerves that help with vasoconstriction, which is the narrowing of the blood vessels. The nerves make the skin on our fingers wrinkle. The wrinkles act like threads on tires. This allows us to get a better grip on things in water. Our ancestors may have benefited from wrinkly feet to good footing in wet areas.
Let’s summarize what we have learned:
- Osmosis is when water moves from an area of LOW solute concentration (low osmolarity) to an area of HIGH solute concentration (high osmolarity) through a semipermeable membrane.
- Osmosis is one of the most important ways that plants and animals achieve homeostasis. Keeping the body's conditions stable makes it possible for living things to survive.
- Osmosis plays an important role in the human body, especially in the gastro-intestinal system and the kidneys. Osmosis helps you get nutrients out of food. It also gets waste products out of your blood.
I admit, that was quite a bit of information! Maybe it’s time to drink some water and eat a snack!
Starting Points
- What clue tells you that your body needs water?
- Do you have any products at home that are concentrated? Do you have to make the product more dilute before using it? Why or why not?
- What are the advantages and benefits of selling a product that is concentrated?
- What are the social and economic advantages and disadvantages of having dialysis technology available for people who have impaired kidney function?
- Does your province or territory have a voluntary organ donation? Is this program really necessary if there is existing technology that can help people with kidney failure? Explain.
- What is the difference between osmosis and osmolarity?
- What is a semipermeable membrane? In the human body, what moves through a semipermeable membrane?
- What is the role of osmosis in the small intestines? In the kidneys?
- Describe how a kidney dialysis machine works to support or replace kidney function?
- How has knowledge of osmosis and semi-permeable membranes had applications in the food industry? (Note: This question requires some additional research)
- This article supports teaching and learning of biology and health related to animal cells, digestive system, excretory system, osmosis & diffusion. Concepts introduced include cells, semipermeable membrane, solutes, solvents, equilibrium, homeostasis, chyme, cortex, medulla, nephrons, dialysis,“fight or flight” response and vasoconstriction.
- Prior to reading this article, teachers could provide students with a Vocabulary Preview to engage prior knowledge and introduce new terminology. Ready-to-use reproducibles using the Vocabulary Preview learning strategy are available in [Google doc] and [PDF] formats.
- To consolidate learning after reading the article, teachers could have students complete a Concept Definition Web learning strategy for the concept of osmosis. Ready-to-use reproducibles for this article are available in [Google doc] and [PDF] formats.
Connecting and Relating
- What clue tells you that your body needs water?
- Do you have any products at home that are concentrated? Do you have to make the product more dilute before using it? Why or why not?
Relating Science and Technology to Society and the Environment
- What are the advantages and benefits of selling a product that is concentrated?
- What are the social and economic advantages and disadvantages of having dialysis technology available for people who have impaired kidney function?
- Does your province or territory have a voluntary organ donation? Is this program really necessary if there is existing technology that can help people with kidney failure? Explain.
Exploring Concepts
- What is the difference between osmosis and osmolarity?
- What is a semipermeable membrane? In the human body, what moves through a semipermeable membrane?
- What is the role of osmosis in the small intestines? In the kidneys?
- Describe how a kidney dialysis machine works to support or replace kidney function?
Nature of Science/Nature of Technology
- How has knowledge of osmosis and semi-permeable membranes had applications in the food industry? (Note: This question requires some additional research)
Teaching Suggestions
- This article supports teaching and learning of biology and health related to animal cells, digestive system, excretory system, osmosis & diffusion. Concepts introduced include cells, semipermeable membrane, solutes, solvents, equilibrium, homeostasis, chyme, cortex, medulla, nephrons, dialysis,“fight or flight” response and vasoconstriction.
- Prior to reading this article, teachers could provide students with a Vocabulary Preview to engage prior knowledge and introduce new terminology. Ready-to-use reproducibles using the Vocabulary Preview learning strategy are available in [Google doc] and [PDF] formats.
- To consolidate learning after reading the article, teachers could have students complete a Concept Definition Web learning strategy for the concept of osmosis. Ready-to-use reproducibles for this article are available in [Google doc] and [PDF] formats.
Learn more
BBC Bitesize Wxplains the three ways substances move around cells: diffusion, active transport, and of course osmosis!
Homeostasis and Negative/Positive Feedback (2017)
This video by the Amoeba Sisters (6:24 min) explains the importance of homeostasis in the human body, with examples of positive feedback and negative feedback.
How do your kidneys work? (2015)
This video by Emma Bryce (3:54 min) details how kidneys balance the amount of fluid in your body, detect waste in your blood, and know when to release the vitamins, minerals, and hormones you need to stay alive.
Why Skin Wrinkles In Water (2014)
Today I Found Out goes into more detail about the different theories there were about why this happens and how they were tested.
References
Eden. (2017) A Comprehensive Break Down of Nephron Functioning into Six Easy Steps! http://blog.cambridgecoaching.com/a-comprehensive-break-down-of-nephron-functioning-into-six-easy-steps
Molnar, C. and Gair, J. (2019) 11.1 Homeostasis and Osmoregulation. CONCEPTS OF BIOLOGY – 1ST CANADIAN EDITION. https://opentextbc.ca/biology/chapter/11-1-homeostasis-and-osmoregulation/
National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center. (2017) Your Digestive System & How it Works https://www.niddk.nih.gov/health-information/digestive-diseases/digestive-system-how-it-works
Reasoner, A. (n.d.) Teaching Osmosis and Diffusion through Kidney Dialysis. https://teachers.yale.edu/curriculum/viewer/initiative_11.07.07_u
Thompson, V. (2016, September 29) How Does Osmosis Occur in the Digestive System? https://education.seattlepi.com/osmosis-occur-digestive-system-4874.html
University of California Los Angeles - Chemistry & Biochemistry. (n.d.) Aqueous Solutions - Molarity. https://www.chem.ucla.edu/~gchemlab/soln_conc_web.htm