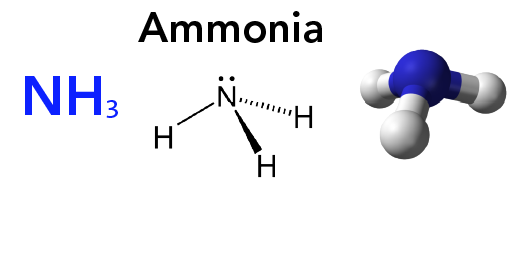
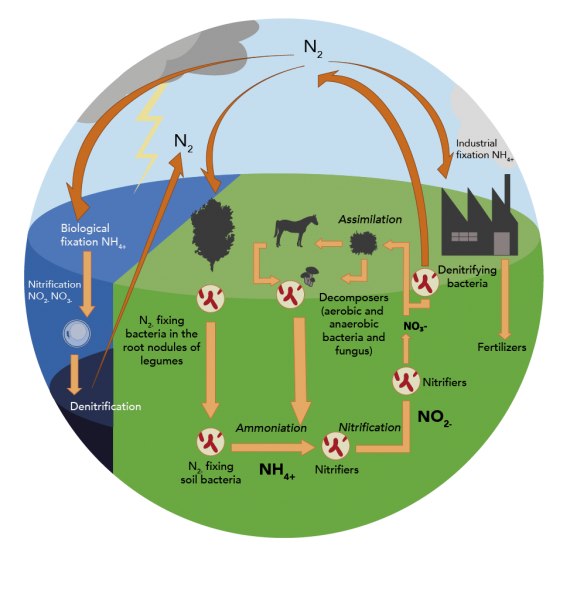
Understanding the Nitrogen Cycle

Nitrogen symbol (ollomy, iStockphoto)

Nitrogen symbol (ollomy, iStockphoto)
7.83
How does this align with my curriculum?
Curriculum Alignment
ON
11
Environmental Science, Grade 11, University/College (SVN3M)
Strand D: Sustainable Agriculture and Forestry
AB
10
Knowledge and Employability Science 10-4 (2006)
Unit D: Investigating Matter and Energy in Environmental Systems
AB
10
Science 14 (2003, Updated 2014)
Unit D: Investigating Matter and Energy in Environmental Systems
BC
9
Science Grade 9 (June 2016)
Big Idea: The biosphere, geosphere, hydrosphere, and atmosphere are interconnected, as matter cycles and energy flows through them.
NU
10
Knowledge and Employability Science 10-4 (2006)
Unit D: Investigating Matter and Energy in Environmental Systems
NU
10
Science 14 (2003, Updated 2014)
Unit D: Investigating Matter and Energy in Environmental Systems
YT
9
Science Grade 9 (British Columbia, June 2016)
Big Idea: The biosphere, geosphere, hydrosphere, and atmosphere are interconnected, as matter cycles and energy flows through them.
NT
10
Knowledge and Employability Science 10-4 (Alberta, 2006)
Unit D: Investigating Matter and Energy in Environmental Systems
NT
10
Science 14 (Alberta, 2003, Updated 2014)
Unit D: Investigating Matter and Energy in Environmental Systems
BC
11
Earth Sciences 11 (June 2018
Big Idea: The transfer of energy through the atmosphere creates weather and is affected by climate change.
ON
11
Environmental Science, Grade 11, University/College (SVN3M)
Strand B: Scientific solutions to Contemporary environmental Challenges
ON
11
Environmental Science, Grade 11, University/College (SVN3M)
Strand C: Human Health and the environment
YT
11
Earth Sciences 11 (British Columbia, June 2018
Big Idea: The transfer of energy through the atmosphere creates weather and is affected by climate change.
AB
6
Science 6 (2023)
Earth Systems: Understandings of the living world, Earth, and space are deepened by investigating natural systems and their interactions.
AB
11
Knowledge and Employability Science 20-4 (2006)
Unit A: Applications of Matter and Chemical Change
BC
7
Science Grade 7 (June 2016)
Big Idea: Elements consist of one type of atom, and compounds consist of atoms of different elements chemically combined.
BC
9
Science Grade 9 (June 2016)
Big Idea: The electron arrangement of atoms impacts their chemical nature.
NU
9
Knowledge and Employability Science 9 (Alberta, Revised 2009)
Unit B: Matter and Chemical Change
NU
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit A: Applications of Matter and Chemical Change
YT
7
Science Grade 7 (British Columbia, June 2016)
Big Idea: The electromagnetic force produces both electricity and magnetism.
YT
9
Science Grade 9 (British Columbia, June 2016)
Big Idea: The electron arrangement of atoms impacts their chemical nature.
NT
9
Knowledge and Employability Science 9 (Alberta, Revised 2009)
Unit B: Matter and Chemical Change
NT
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit A: Applications of Matter and Chemical Change