Describing and Classifying Matter

Colourful liquids and periodic table (peepo, iStockphoto)

Colourful liquids and periodic table (peepo, iStockphoto)
How does this align with my curriculum?
Learn about the physical and chemical properties of matter.
Describing Matter
Each type of matter has its own unique properties. A property is a characteristic we can use to identify matter. We can use the properties of matter to know that wood is wood and gold is gold. The properties of matter fall into two categories. The first is physical properties. The second is chemical properties.
Physical Properties
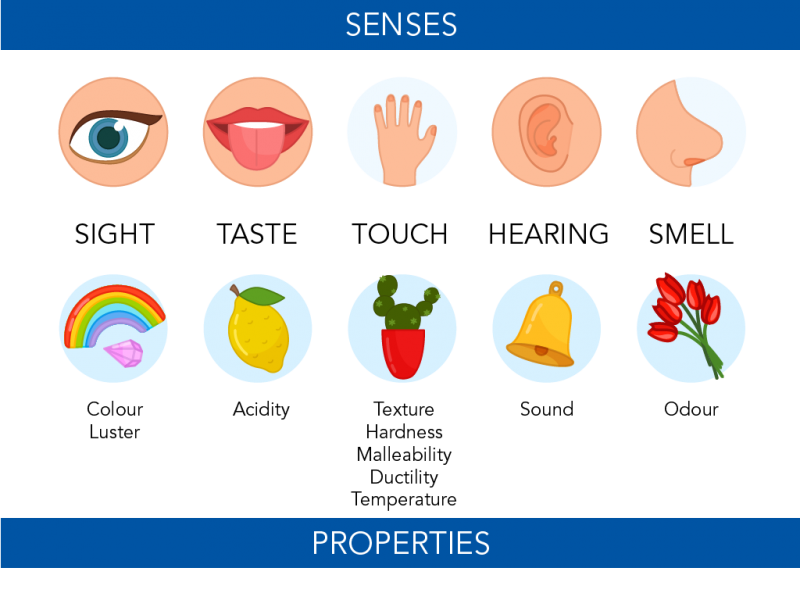
A physical property is a way to describe the physical form of matter. We can identify some of these with our senses. Colour can be seen. So can luster. This is how shiny or reflective something is. Odour can be smelled. Some things, like acidity, can be tasted. Texture can be felt through touch. So can hardness and temperature. A person can try to flatten things to test their malleability. Or they can try to stretch things out to test their ductility.
Image - Text Version
Shown is a colour illustration showing five senses and five examples of properties they can identify.
Each illustration is on a pale blue circle against a cream background. The top row is labelled "Senses". The bottom row is labelled "Properties."
Starting on the left, the first illustration shows a single blue eye. This is labelled "Sight." Below is an illustration of a rainbow and a gleaming pink gem. This is labelled "Colour" and "Lustre."
The next illustration on the top is a mouth with the tongue sticking out. This is labelled "Taste." Below is a yellow lemon with one leaf, labelled "Acidity."
The third is a hand on a white background. This is labelled "Touch." Below is a green cactus plant, with prickles, in a red pot. This is labelled "Texture, Hardness, Malleability, Ductility, Temperature."
The fourth is one ear. This is labelled "Hearing." Below is a shiny, gold-coloured bell labelled "Sound."
The fifth illustration is a nose, shown from the side. This is labelled "Smell." Below is a bunch of red tulip flowers. This is labelled "Odour."
Some properties can’t be identified through the senses. But they can be measured. Scientists do this without changing the matter. These properties include boiling point, melting point, electrical conductivity, magnetism and density.
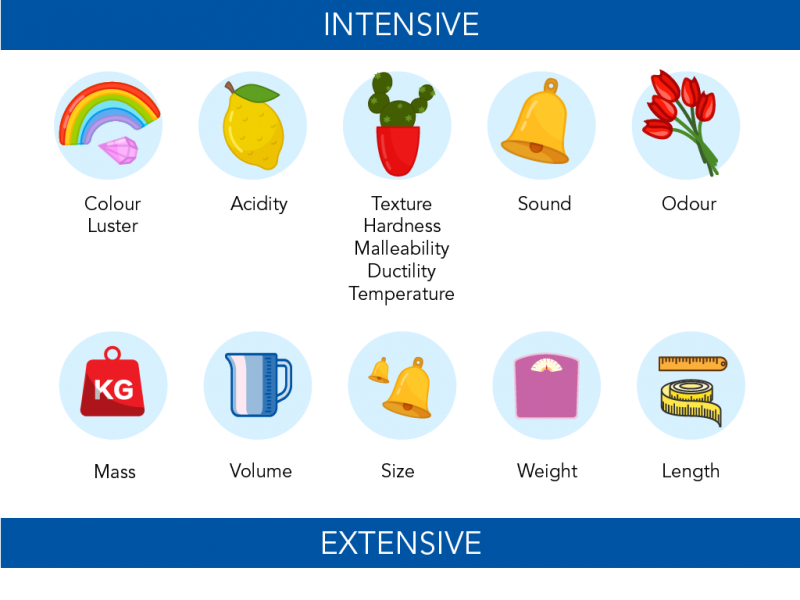
Physical properties can be intensive or extensive.
An intensive property does not depend on the amount of matter. Colour, odour, density and melting point are intensive properties. An extensive property depends on the amount of matter. These include things like mass, volume and length.
Image showing extensive and intensive properties of matter (Let’s Talk Science using images by Alena Igdeeva, bortonia and erhui1979 via iStockphoto).
Image - Text Version
Shown is a colour illustration showing five examples of intensive properties and five examples of extensive properties.
Each illustration is on a pale blue circle against a cream background. The top row is labelled "Intensive". The bottom row is labelled "Extensive."
Starting on the top left, the first illustration shows a rainbow and a gleaming pink gem. This is labelled "Colour" and "Luster."
The second is a yellow lemon with one leaf. It is labelled "Acidity."
The third is a green cactus plant with prickles, in a red pot. It is labelled "Texture, Hardness, Malleability, Ductility, Temperature."
The fourth is a shiny, gold-coloured bell. This is labelled "Sound."
The fifth is a bunch of red tulip flowers. This is labelled "Smell."
On the bottom row, starting on the left, the first illustration is a red weight with "KG" written on the front. This is labelled "Mass."
The second is a blue jug. This is labelled "Volume."
The third shows two bells. One large and one small. This is labelled "Size."
The fourth shows a purple bathroom scale. This is labelled "Weight."
The last illustration shows a ruler and a tape measure. This is labelled "Length."
Chemical Properties
A chemical property describes how likely it is the matter will go through a chemical reaction. Here are some examples of chemical properties:
Flammability
Flammability Is the ability of matter to burn or combust. Things that are flammable can ignite easily and burn quickly. Flammable matter is often called fuel.
Wood, gasoline and wax are all flammable.
Corrosiveness
Corrosiveness is the ability of matter to ‘eat away’ another substance. It is important to wear safety equipment to protect your skin and eyes when using corrosive materials.
Corrosive materials include strong acids and bases. Hydrochloric acid and bleach are both corrosive.
Toxicity
Is the ability of a material to cause damage to living things. Toxic materials cause harm when inhaled, swallowed or contact skin.
Lead, mercury, and chlorine gas are all toxic.
Classifying Matter
There are different ways to classify matter. One way is to classify it as a pure substance or a mixture.
Pure Substances
A pure substance is the same throughout. It can’t be separated into other substances, or transformed into a new substance.
The physical properties of a pure substance never change. Water is a pure substance. So, the boiling point of water is always 100 degrees Celsius at a pressure of 101.3 kilopascals.
Pure substances only contain one type of element or compound.
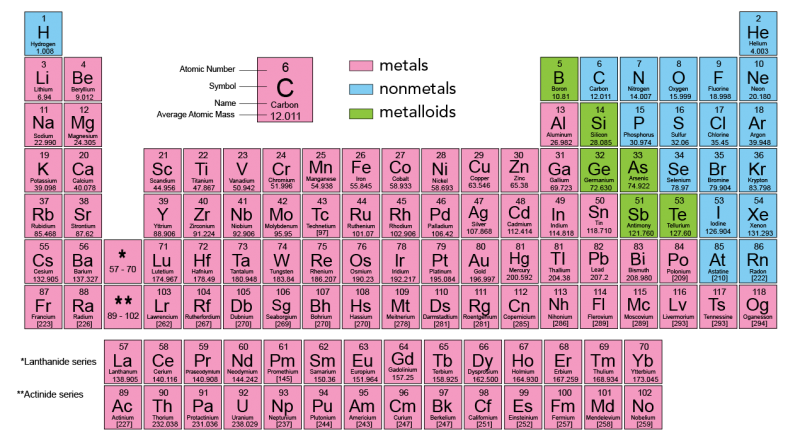
Elements
Elements are a type of pure substance. They contain only one type of atom. These include carbon (C), silver (Ag) and gold (Au). Scientists have organized the elements into a chart called The Periodic Table of Elements.
Click here to access screen reader Accessible Periodic Tables
Each element has a specific set of properties. These are called characteristic properties. Characteristic properties are all extensive. These include density, melting point, boiling point, electronegativity and atomic weight.
Did you know?
Currently, there are 118 known elements. Several of these elements were discovered during your lifetime!
Image - Text Version
Shown is a colour diagram of a molecule labelled O2 and a molecule labelled CO2 on a cream background.
The O2 diagram shows two blue spheres labelled "O", side-by-side. These are joined by two thick, black, horizontal lines.
The CO2 diagram has a red sphere labelled "C" in the centre, with one blue "O" sphere on either side. These are joined by two sets of thick, black, horizontal lines.
Compounds also have a fixed ratio. For example, a water molecule always has two hydrogen atoms (H) and one oxygen atom (O).
Compounds can be broken down into their individual elements. This is done through chemical reactions. For example, water can be broken down into hydrogen and oxygen using electrolysis.
The properties of a compound are different from the properties of each element it contains. Water has different properties than hydrogen or oxygen.
Mixtures
Mixtures are a physical combination of two or more pure substances. When they are mixed together, each pure substance keeps its own properties. For example, salt water does not have the same properties as either salt or water. It is not a white crystalline powder like salt. And it freezes at a lower temperature than water.
Mixtures can be either homogeneous or heterogeneous.
A homogeneous mixture has the same composition throughout. Salt water is a good example of a homogeneous mixture. This is because dissolved salt is spread evenly through the mixture.
Another name for a homogeneous mixture is a solution. Solutions can be made of liquids, gases or solids. Air is a solution made of gases. Alloys are solutions made of metals. These include bronze and brass.
Image - Text Version
Shown are three colour photographs arranged in a row. The first is a glass and a bottle of milk, second is a hot air balloon, and third is a brass bell. The milk containers are clear glass, sitting on a marble table with a teal background. The camera is looking up at the yellow, orange, red and blue striped balloon, flying in the bright blue sky. The bell is a pale gold colour and gleaming in the sun. It is mounted on a curved white wall with deep blue sky in the background.
A heterogeneous mixture is not the same throughout. The individual parts can be seen in these mixtures. Vegetable soup is a heterogeneous mixture. Each spoonful might include different vegetables, in different amounts. Heterogeneous mixtures also include things like salad dressing or mixed nuts.
Learn More
What's Matter? (2015)
This video (3:30 min.) from Crash Course Kids defines matter and shows an experiment you can do at home.
Science Bits: Pure Substances and Mixtures (2014)
This short video (2:08 min.) from Science Bits is an overview of pure substances and mixtures.
Physical and Chemical Properties (2023)
This video (2:36 min.) from MooMooMath and Science explains the difference between physical and chemical properties, along with examples.
Extensive vs Intensive Properties of Matter - Explained (2015)
This video (6:03 min.) from Chem Academy explores several examples of extensive and intensive properties and works through sample problems.
References
Canadian Centre for Occupational Health and Safety. (2018). Pictograms.
Helmenstine, A.M. (Updated 2019, Dec. 4). The Difference Between Intensive and Extensive Properties. Thought Co.
Science Buddies. (2016, April 7). Splitting Water. Scientific American.