Introduction to the Particle Theory of Matter
Cube overflowing with spheres representing particles (akinbostanci, iStockphoto)

Cube overflowing with spheres representing particles (akinbostanci, iStockphoto)
How does this align with my curriculum?
Learn about how the Particle Theory helps us understand matter.
Particle Theory of Matter
Matter is anything that has mass and takes up space. It is a general name we call all the physical things around us. Matter includes things so tiny humans can’t see them with their eyes.
The Particle Theory of Matter is a scientific model. A scientific model is a way of illustrating ideas, objects and processes so they’re easier to understand. Scientists use models to explain things that can’t be seen without special equipment. One of these things is an individual atom.
The Particle Theory of Matter helps us think about how matter behaves. It also helps us explain why different matter has different properties. It includes these key ideas:
- All matter is made of tiny particles. These particles are either individual atoms, or groups of atoms called molecules.
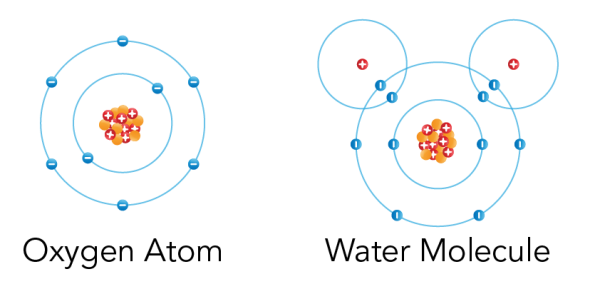
Image - Text Version
Shown is a colour diagram of two different atoms with blue spheres shown along concentric rings around a clump of red and orange spheres at the centre. The atom on the left is labelled "Oxygen Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Eight blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom on the right is labelled "Water Molecule." The main part of this diagram has the same amount of spheres and circles as the first. But there are two additional red spheres with plus signs. These are in the top left and right corners. Each one is surrounded by an additional blue circle with one blue sphere with a minus sign. These overlap the edges of the larger circle below.
Did you know?
Any particle smaller than an atom is called a subatomic particle. Protons, neutrons and electrons are all subatomic particles.
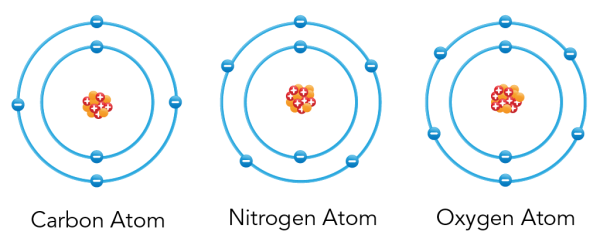
- Atoms of the same element are the same. Atoms of different elements are different. So, all of the atoms in carbon are the same. But the atoms in nitrogen and oxygen are different from carbon atoms.
From left to right: Bohr atomic models of carbon, nitrogen and oxygen (Let’s Talk Science using an image by VectorMine via iStockphoto)
Image - Text Version
Shown is a colour diagram of three different atoms with blue spheres shown along concentric rings around a clump of red and orange spheres at the centre. The atom on the left is labelled "Carbon Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Six blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom in the middle is labelled "Nitrogen Atom." This has a clump of orange spheres and red spheres with plus signs at the centre. Seven blue spheres with minus signs are neatly arranged along two concentric blue circles around it. The atom on the right is labelled "Oxygen Atom" This has a clump of orange spheres and red spheres with plus signs at the centre. Eight blue spheres with minus signs are neatly arranged along two concentric blue circles around it.
- Particles are attracted to each other by forces. In some kinds of matter, like a diamond, this force is very strong. In other kinds of matter, like orange juice, the force is weaker.
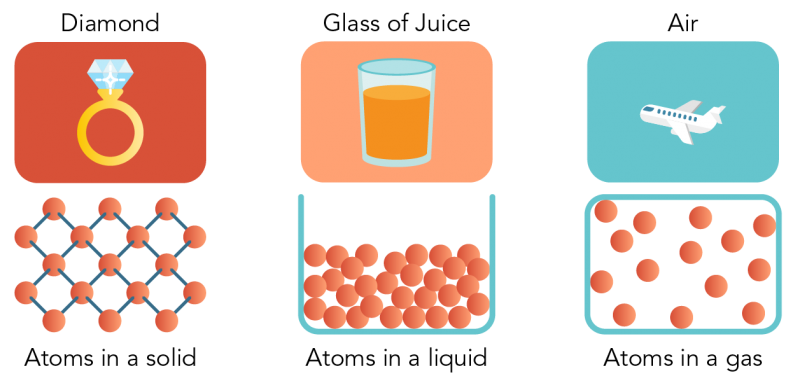
- Particles of matter have spaces between them. In a gas, there are large spaces between them. In a liquid they are closer together. In a solid, the particles are packed so close they can hardly move.
Image - Text Version
Shown is a colour illustration of three different kinds of matter above diagrams of their atoms. Starting on the left, the first illustration is labelled "Diamond." It shows a gold ring topped with a large, clear gem on a red background. Below, atoms are represented as pink spheres connected by small blue sticks. These are arranged in a neat diamond pattern, like a chain link fence. Text below reads "Atoms in a solid." In the middle is an illustration labelled "Glass of Juice." It shows a clear glass of orange juice against a pink background. Below, atoms are piled together, filling the bottom of an open blue box shape that resembles the glass. Some spheres appear to be touching each other, but there is space between others. Text below reads "Atoms in a liquid." On the right is an illustration labelled "Air." This shows a white airplane flying in a blue sky. Below, atoms float loosely in a blue rectangle, with lots of space between them. Text below reads "Atoms in a gas."
- Particles are always moving at any temperature above -273.15 degrees Celsius. But the human eye can’t see them move.
Did you know?
-273.15 degrees Celsius is also 0 kelvin (0 K). This temperature is called absolute zero.
- The faster particles move, the warmer they get. So, the molecules in hot water are moving faster than the ones in cold water.
Image - Text Version
Shown is a colour diagram illustrating the speed and temperature of particles along a scale from colder to warmer. A thick horizontal stripe runs across the bottom edge. This is blue on the left side, and gradually changes to purple, then pink on the right. The left end is labelled "Slower" on top, and "Colder" below. The right end is labelled "Faster" on top, and "Warmer" below. Three black circles are spaced out along the scale. Each one contains round, coloured circles to represent particles. These have translucent tails that indicate the speed of their movement. The left circle has blue particles with short, wide tails. These match the blue, colder end of the scale. The middle circle has purple particles with longer, narrower tails. These match the middle part of the scale. The right circle has pink particles with even longer, narrower tails. These match the pink, warmer end of the scale.
Learn More
Explore the optimal environmental conditions for human life. How do you think your classroom conditions compare to those on the International Space Station? Free project for grades 6-9 students.
The Particle Theory of Matter Quiz
See how well you know the particle theory. Try this short quiz by Science Source.
What's Matter?
This video (3:30 min) by Crash Course Kids explains three states of matter: Solid, Liquid, and Gas. There is also a quick experiment you can do at home to prove that air is matter.
GCSE Physics - Particle Theory & States of Matter #25 (2020)
This video (4:33 min.) from Cognito covers particle theory, how substances change from one state to another, and the idea that density doesn't change when substances change state.
References
BBC Bitesize. (n. d.) Kinetic particle theory - Kinetic particle theory and state changes - GCSE Physics (Single Science) Revision - Other.
EduMedia. (n. d.) Understanding Matter and Energy, Pure Substances and Mixtures
Petersen, D. (2019 March 26). How to teach states of matter and particle theory - Royal Society of Chemistry