Moles and Molarity: Counting and Chemistry from Donuts to Soap

A mole and the number of atoms or molecules in a mole (nwbob, iStockphoto)

A mole and the number of atoms or molecules in a mole (nwbob, iStockphoto)
How does this align with my curriculum?
| Grade | Course | Topic |
|---|
To count tiny things like atoms and molecules, scientists use a measurement called the mole.
Imagine you are working in a donut shop. A customer comes in and asks you for a dozen donuts. There are twelve to a dozen, so you count out twelve donuts. Very quickly, you are finished serving the customer.

Later, another customer comes in with an unusual order. He doesn't want a standard box of twelve donuts. He wants a special order of twenty-four donuts decorated with ten thousand sprinkles to celebrate having ten thousand followers on Instagram.
This is a bit of an outlandish request, but your manager says to do it.
So, there you are, counting out individual sprinkles. By sprinkle 110, you start to think there has to be a better way. An idea occurs to you: "Can I use the kitchen scale to do the counting for me?"
So you take the 110 sprinkles you've already counted and put them on the scale. It reads 0.19 grams. Now that you know how much 110 sprinkles weigh, you can calculate how much 10,000 sprinkles will weigh.
(0.19 g)/(110 sprinkles) x 10 000 sprinkles = 17.27 g of sprinkles
You carefully weigh out 17.27 grams of sprinkles. You’re pretty pleased with how much time you have saved by not counting sprinkles!
What is a mole? (and we don’t mean the animal)
Scientists use a similar method to determine how many atoms or molecules there are in a substance. They need to do this to combine the proper amounts of chemicals for chemical reactions. Unlike sprinkles though, it’s physically impossible to count atoms or molecules. They’re just too small!
To solve this problem, scientists use a unit of measurement that connects the amount of something to the corresponding number of atoms or molecules. Donuts usually come by the dozen, and there are 12 donuts in a dozen. Similarly, atoms and molecules can be measured by the mole (symbol: mol). There are 602 200 000 000 000 000 000 000 units per mole.
That’s a lot of zeros! To work with large numbers like this, people use scientific notation. This is a short way of writing a large number. Using scientific notation reduces the chance of making a mistake!
To use scientific notation:
- count from the decimal place in the number until there is only one digit remaining to the left
- move the decimal to this location
- multiply by 10 to the power of the number of decimal places you counted to
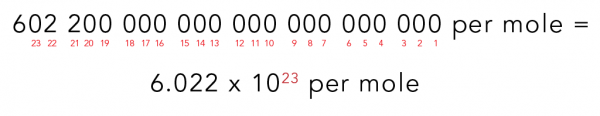
So, the scientific notation used to calculate a mole is 6.022 x 1023.
Now, let's get an idea of how big a mole is by figuring out the mass of a mole of sprinkles:
(0.19 g)/(110 sprinkles)*
x
6.022 x 1023 sprinkles/mole†
=
1.04 x1021 g/mol‡
x
(1 kg)/(1000 g)§
=
1.04 x1018 kg/mol¶
- *110 sprinkles had a mass of 0.19 grams
- †6.022 x 1023 is the number of objects in a mole
- ‡1.04 x1021 g/mol is the mass of a mole of sprinkles in grams
- §(1 kg)/(1000 g) is the conversion from grams to kilograms. There are 1000 grams in a kilogram
- ¶1.04 x1018 kg/mol is the mass of a mole of sprinkles in kilograms
A mole of sprinkles is over 260 000 times the mass of food produced for human consumption each year on Earth! So, you are never going to see a mole of sprinkles!
Did you know?
The number of units in a mole is so large, you would never have enough time to count it out. In fact, if every human who ever lived spent their whole lives counting objects one by one, all of their counting added together would still not reach one mole.
How does using moles help scientists?
Atoms and molecules are much smaller than sprinkles. The mole exists to help scientists work with such tiny particles.
For example, diamonds are made of carbon. A mole of carbon atoms has a mass of about 12 grams. The Tiffany Diamond, one of the largest diamonds in the world, weighs about 26 grams. This is more than two moles of carbon. So, a mole of atoms is a straightforward quantity for people to measure.

The number of particles in a mole, 6.022x1023, is often called Avogadro's constant or Avogadro’s number. It is named after Amedeo Avogadro, an Italian physicist.
So when would anyone use Avogadro's constant? Likely when making a solution that involves many chemical reactions. When performing a reaction, you combine appropriate numbers of molecules of each chemical based on the number of moles required.
To determine how much of a solution to use, chemists use molarity. That’s the number of moles of a chemical in a litre of solution.
A concentration of 1 mole in 1 litre is called 1 molar and abbreviated 1 M or 1 mol/L.
To see this in action, let’s imagine you are a chemist trying to make soap. You need to react vegetable oil or animal fat with sodium hydroxide to produce soap. Imagine you had measured some oil and determined it contained 0.2 moles of fat molecules called triglycerides.
In the soap-making reaction, 1 mole of triglycerides reacts with 3 moles of sodium hydroxide. So how much 12 molar sodium hydroxide solution would you need to react with the vegetable oil containing 0.2 moles of triglycerides?

Step One: Calculate moles of sodium hydroxide needed
Multiply the moles of triglycerides needed by the ratio of reactants (the number of moles of sodium hydroxide divided by 1 mol of triglycerides):
0.2 mol of triglyceride x (3 mol of sodium hydroxide)/(1 mol of triglycerides) = 0.6 mol of sodium hydroxide
Step Two: Calculate volume of 12 molar sodium hydroxide needed
Number of moles = molarity x volume
Rearrange this to find the formula for calculating volume:
Volume = number of moles / molarity
= 0.6 mol of sodium hydroxide / 12 mol/L = 0.05 L of 12 molar sodium hydroxide
Step Three: Convert litres to millilitres
For small amounts, like those used in making soap, millilitres is a more useful measurement than litres. Remember, 1L = 1 000ml.
0.05 L x 1 000 mL/L = 50 mL
50 mL of 12 molar sodium hydroxide would be required.
That was a lot of information! Let’s sum up the key points:
- A mole is a specific number used to describe very large amounts of very tiny atoms or molecules.
- In chemistry, knowing how many moles there are in a solution is important for performing reactions, like making soap.
- Concentrations in molarity, or moles in a litre, can help you determine the correct amount of a solution needed.
Did you know?
October 23rd (10/23!) from 6:02 am to 6:02 pm is Mole Day. It’s a day to celebrate chemistry!
Starting Points
- Have you ever tried measuring something that is very small? What problems did you encounter?
- Have you ever made a solution at home? What was that solution made of? What type of measuring did you do to make the solution, if any?
- What are some applications of chemical solutions that are found in industry and technology?
- What are the benefits of being able to change the molarity of a solution?
- Define a mole.
- What is Avogadro’s constant?
- What happens to the molarity of a solution if it is diluted?
- Write the following numbers in scientific notation:
- 4 703 700 000 000 000 000
- 99 900 000
- What is the value of scientific notation?
- Can you think of other units of measurement that scientists use for measuring items that are very small or very large in terms of size or distance?
- How do manufacturers of laundry detergent advertise the benefits of more concentrated detergent solutions? What other products are you aware of that use concentration as a selling feature?
- This article supports teaching and learning of Chemistry and Mathematics related to Molarity & Stoichiometry and Number Sense & Numeration. Concepts introduced include mole (mol), scientific notation, carbon, solution, molarity and molar (1M or mol/L).
- This article could be read prior to a hands on chemistry lab involving mixing solutions.
- After reading the article, teachers could have students complete a Concept Definition Web learning strategy to explore the concept of mole (mol). Ready-to-use reproducibles for this article are available in [Google doc] and [PDF] formats.
- Teachers could also have students work on solving solutions problems, like the problem outlined in the article, to follow up this reading and reinforce the concepts and calculations presented.
Connecting and Relating
- Have you ever tried measuring something that is very small? What problems did you encounter?
- Have you ever made a solution at home? What was that solution made of? What type of measuring did you do to make the solution, if any?
Relating Science and Technology to Society and the Environment
- What are some applications of chemical solutions that are found in industry and technology?
- What are the benefits of being able to change the molarity of a solution?
Exploring Concepts
- Define a mole.
- What is Avogadro’s constant?
- What happens to the molarity of a solution if it is diluted?
- Write the following numbers in scientific notation:
- 4 703 700 000 000 000 000
- 99 900 000
Nature of Science/Nature of Technology
- What is the value of scientific notation?
- Can you think of other units of measurement that scientists use for measuring items that are very small or very large in terms of size or distance?
Media Literacy
- How do manufacturers of laundry detergent advertise the benefits of more concentrated detergent solutions? What other products are you aware of that use concentration as a selling feature?
Teaching Suggestions
- This article supports teaching and learning of Chemistry and Mathematics related to Molarity & Stoichiometry and Number Sense & Numeration. Concepts introduced include mole (mol), scientific notation, carbon, solution, molarity and molar (1M or mol/L).
- This article could be read prior to a hands on chemistry lab involving mixing solutions.
- After reading the article, teachers could have students complete a Concept Definition Web learning strategy to explore the concept of mole (mol). Ready-to-use reproducibles for this article are available in [Google doc] and [PDF] formats.
- Teachers could also have students work on solving solutions problems, like the problem outlined in the article, to follow up this reading and reinforce the concepts and calculations presented.
Learn more
How big is a mole? (Not the animal, the other one.) (2012)
This video (4:32 min.) from Daniel Dulek explains how the mole was developed, and how big it really is.
Chemistry Lesson: The Mole (Avogadro's Number) (2013)
This video (9:48 min.) from GetChemistryHelp discusses how the mole is a unit of measure for the amount of a chemical substance. 6.022x1023 particles, which may be atoms, molecules, formula units, or other particles.
An article from BBC Bitesize about how to use moles to create a balanced chemical equation.
How big is a mole? (2010)
In this article on Wired for Mole Day, Rhett Allain tries to show us moles of various substances.
References
Capri, A. (2003). The mole and atomic mass. Visionlearning.
Criswell, B. (2008). Teaching Avogadro’s hypothesis and helping students to see the world differently. Journal of Chemical Education, 85(10), 1372. DOI: 10.1021/ed085p1372
Helmenstine, A. M. (2019). What is a mole in chemistry? ThoughtCo.
LibreTexts. (2019). The mole and Avogadro’s constant.