What Causes Hot Things to Glow?

Casting hot iron (dt03mbb, iStockphoto)

Casting hot iron (dt03mbb, iStockphoto)
How does this align with my curriculum?
| Grade | Course | Topic |
|---|
Getting electrons excited by heat can cause certain materials to give off visible light when heated - like filaments in light bulbs.
A stovetop burner glows red when it’s set to maximum. An old-fashioned light bulb gets too hot to handle after it’s on for a while. Certain chemicals, when set on fire, produce brilliant colours. But why do heat and light seem to go together?
The answer lies in the smallest unit of matter, the atom. At the centre of the atom is the nucleus, which contains positively-charged protons and uncharged neutrons. Negatively-charged electrons exist in shells, which are the areas that surround the nucleus. A shell is also sometimes called an energy level.
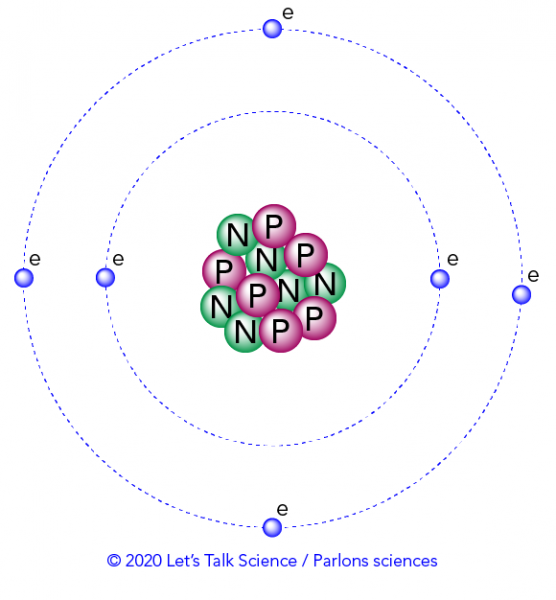
How can heat be transformed into light?
Electrons are what allow atoms to react with each other in chemical reactions. They are also what cause certain compounds to emit light when heated.
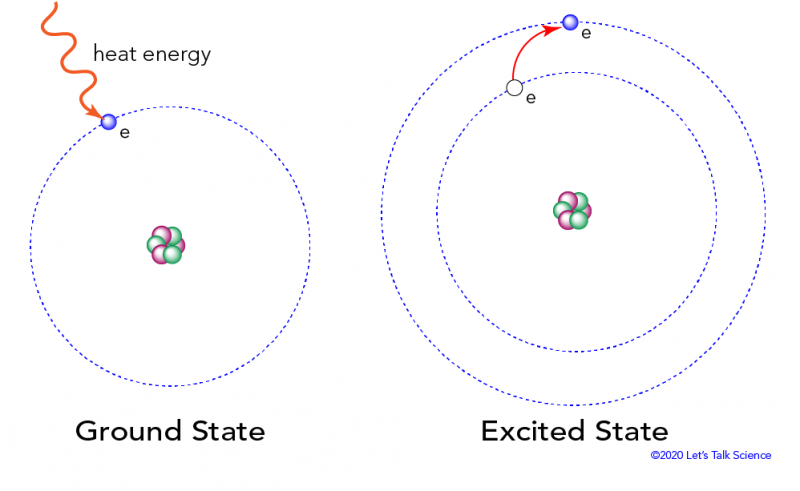
Each electron has a shell that is its “home base.” When it is in that shell, it is said to be in its ground state. The ground state is the state of lowest energy for that electron. At room temperature, most electrons are in their ground state.
When heat is transferred to an atom, it starts to vibrate more quickly. This vibration is a form of kinetic energy. Some of the kinetic energy is transferred to the electrons around the nucleus. This makes them “jump” from their usual shell into a shell that is further away from the nucleus. When an atom’s electrons move out of place like this, it is said to be in an excited state.
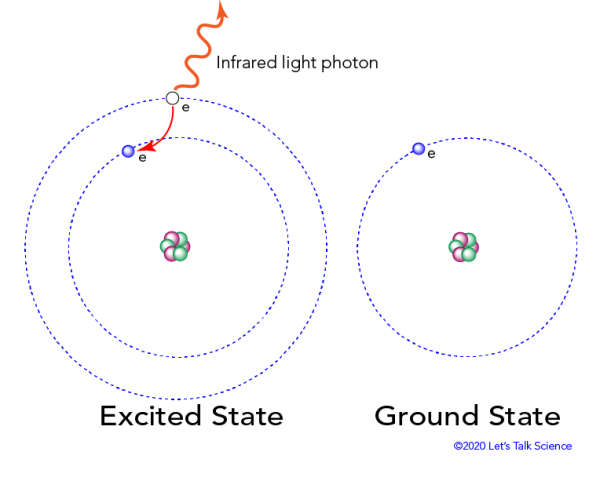
This excited state is very unstable, and the electron quickly falls back down to its normal shell, and ground state. When this happens, the electron releases the extra energy it had gained in the form of infrared light photons. These photons are invisible to the human eye.
How do light bulbs make light?
The ability of electrons to convert heat to infrared light can be used in lots of cool ways! The most famous example is an incandescent light bulb. Inside the glass bulb is small piece of metal (filament) made of tungsten. An electrical current is passed through the filament. But it doesn’t pass through easily. This is because the filament acts as a resistor. Resistors get hot when electricity passes through them. So hot, in fact, that it gives off enough energy to cause the filament to glow. This is the same reason that the cooking elements on an electric stove glow red when they heat up.
Did you know?
The tungsten filament in an incandescent light bulb heats up to over 2 000 degrees Celsius when in use!
One of the reasons incandescent bulbs are being phased out is because the majority of the energy used – up to 95 percent – is spent heating up the filament, rather than producing light. That is a waste of energy!
How do fireworks make light?
Fireworks also work on the same principle. Fireworks are made from different compounds containing metals. When heated, these compounds produce different colours of light. Metals that absorb higher energies will release shorter wavelengths of light (such as blue light) when their electrons return to their ground state. Metals that absorb lower energies will release longer wavelengths of light (such as red light) when their electrons return to their ground state.

Did you know?
Colour production in fireworks is a fine art, and fireworks technicians often guard their colour recipes very closely!
So there you have it. Heat and light go hand in hand to create some amazing things. From lighting up our homes to lighting up the night!
Starting Points
- What items have you observed that glow when they are heated?
- Have you watched fireworks before? How do you think the different colours are produced?
- Do you have a preference for the type of light source you use to read or study? What features are important to you in terms of light quality?
- Describe some of the key impacts of the invention of the incandescent light bulb at the end of the 19th century.
- What things have driven the development of different types of light bulbs?
- What is an electron shell?
- At the atomic level, how is heat energy transformed to light energy?
- How do fireworks produce different colours?
- What different light bulbs are described in the video? How are they similar in terms of how they produce light? How are they different?
- How has the development and production of modern fireworks relied on innovations in science and technology?
- What is the role of a gaffer in the film industry? Research how this title was derived? How was the film industry affected by the development of new lighting technology in the early 20th century?
- This article and embedded video can be used to support teaching and learning of Chemistry and Technology & Engineering related to atomic structure, heat transfer, metals and visible light. Concepts introduced include atoms, nucleus, protons, neutrons, electrons, shells, energy levels, ground state, heat transfer, excited state, infrared light photons, filament and wavelengths.
- Prior to reading this article, teachers could have students complete a Vocabulary Preview learning strategy to engage prior knowledge about atomic structure and introduce new terminology. Ready-to-use Vocabulary Preview reproducibles for this article are available in [Google doc] and [PDF] formats.
- While viewing the embedded video in this article, teachers could have students use an Importance Line learning strategy to highlight and organize the key points from the video. Ready-to-use Importance line reproducibles for this article are available in [Google doc] and [PDF] formats.
- Alternatively, after reading the article and viewing the video, teachers could have students create a graphic organizer to summarize the how light is produced using the various light bulbs and light technologies outlined in the embedded video.
Connecting and Relating
- What items have you observed that glow when they are heated?
- Have you watched fireworks before? How do you think the different colours are produced?
- Do you have a preference for the type of light source you use to read or study? What features are important to you in terms of light quality?
Relating Science and Technology to Society and the Environment
- Describe some of the key impacts of the invention of the incandescent light bulb at the end of the 19th century.
- What things have driven the development of different types of light bulbs?
Exploring Concepts
- What is an electron shell?
- At the atomic level, how is heat energy transformed to light energy?
- How do fireworks produce different colours?
- What different light bulbs are described in the video? How are they similar in terms of how they produce light? How are they different?
Nature of Science/Nature of Technology
- How has the development and production of modern fireworks relied on innovations in science and technology?
Media Literacy
- What is the role of a gaffer in the film industry? Research how this title was derived? How was the film industry affected by the development of new lighting technology in the early 20th century?
Teaching Suggestions
- This article and embedded video can be used to support teaching and learning of Chemistry and Technology & Engineering related to atomic structure, heat transfer, metals and visible light. Concepts introduced include atoms, nucleus, protons, neutrons, electrons, shells, energy levels, ground state, heat transfer, excited state, infrared light photons, filament and wavelengths.
- Prior to reading this article, teachers could have students complete a Vocabulary Preview learning strategy to engage prior knowledge about atomic structure and introduce new terminology. Ready-to-use Vocabulary Preview reproducibles for this article are available in [Google doc] and [PDF] formats.
- While viewing the embedded video in this article, teachers could have students use an Importance Line learning strategy to highlight and organize the key points from the video. Ready-to-use Importance line reproducibles for this article are available in [Google doc] and [PDF] formats.
- Alternatively, after reading the article and viewing the video, teachers could have students create a graphic organizer to summarize the how light is produced using the various light bulbs and light technologies outlined in the embedded video.
Learn more
Electrons and Photons: Absorption and Transmission of Light (2014)
This video from ABC Education (1:51 min) briefly introduces the different electron-shell models and explains how the absorption of energy can be transmitted as different coloured light.
The Chemistry of Fireworks (2013)
This article from Compound Interest explains the chemistry of fireworks; includes an infographic with examples of the different kinds of metals that produce different firework colours, as well as some of the chemistry behind how these colours are produced. Note that this resource was also used as a reference.
References
Chem4Kids. (n.d.). Always in motion.
Chem4Kids (n.d.). Atoms are building blocks.
Goddard Space Flight Center, National Aeronautics and Space Administration. (n.d.). Background: Atoms and light energy.
Harris, T. (2002). How light bulbs work. HowStuffWorks.
How It Works Team. (2012, June 9). How do atoms emit light?
Woodford, C. (2019, May 23). How do heating elements work? Explain That Stuff.