Introduction to the Atom

Cloud model of an atom (koto_feja, iStockphoto)

Cloud model of an atom (koto_feja, iStockphoto)
How does this align with my curriculum?
Learn about the parts of an atom and its history.
The Atom
According to the Particle Theory of Matter, all matter is made of tiny particles. These particles are either individual atoms or groups of atoms called molecules. There are two main parts to an atom. These are the nucleus and the electrons.
The Nucleus
In the center of each atom is the nucleus. Within the nucleus there are two kinds of particles. Positively-charged particles called protons and particles with no charges called neutrons. The protons give the nucleus a positive charge. For example, a helium atom has 2 protons and 2 neutrons. It has a nuclear charge of +2. A carbon atom has 6 protons and 6 neutrons. It has a nuclear charge of +6.
Image - Text Version
Shown is a colour diagram of the protons and neutrons in two different nuclei.
The left diagram is titled "Helium Nucleus." It is made up of two different types of spheres, arranged in a tight clump. There are two red spheres with white plus signs. These are labelled "Protons: Positively charged particles." There are also two plain orange spheres. These are labelled "Neutrons: Particles with no charge." The whole clump is labelled "2 protons, 2 neutrons: Nuclear charge +2."
The right diagram is titled "Carbon Nucleus." This is made up of six red spheres with plus signs and six orange spheres, in a clump. This diagram is labelled "6 protons, 6 neutrons: Nuclear charge +6."
The number of protons in the nucleus defines the type of atom. For example, all gold atoms have 79 protons and all silver atoms have 47 protons. The protons and neutrons in the nucleus are held together by a force called the strong nuclear force.
Did you know?
Neutrons were not discovered until 1932. They were discovered by scientist James Chadwick.
The Electrons
Moving around the nucleus are tiny, negatively-charged particles called electrons. Electrons are 2 000 times smaller than protons and neutrons. This means that most of the mass of an atom is found in its nucleus.
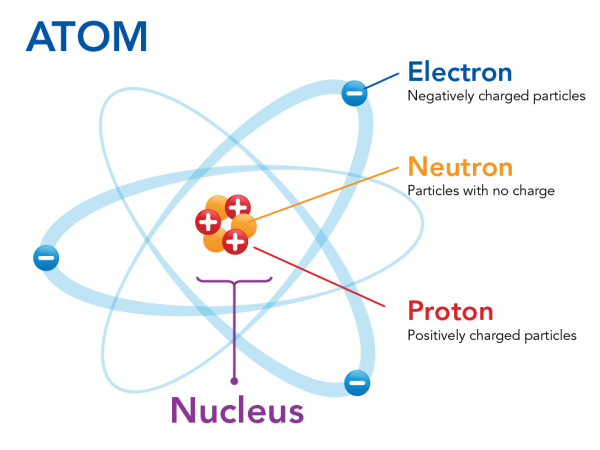
Image - Text Version
Shown is a colour diagram of an atom showing electrons moving around a nucleus. The diagram is titled "Atom" in block letters. In the centre is a clump of three red spheres with plus signs and three orange spheres. One red sphere is labelled "Proton: Positively charged particles." One orange sphere is labelled " Neutron: Particles with no charge." The whole clump is labelled "Nucleus." Around the nucleus are three blue spheres with minus signs. The top one is labelled "Electron: Negatively charged particles." Each electron orbits the nucleus on a separate, pale blue oval path. These are fanned out evenly around the nucleus in the centre.
Electrostatic forces keep the electrons close to the nucleus. These are the forces that pull negatively-charged and positively-charged particles towards each other. But electrons also have kinetic energy. This pushes them away from the nucleus. In a stable atom, these two forces are balanced. This keeps the electrons within certain areas around the nucleus.
In nature, most atoms are stable. A stable atom has the same number of electrons as protons. For example, a gold atom has 79 protons and 79 electrons. Silver has 47 positively-charged protons and 47 negatively-charged electrons. These positive and negative charges cancel each other out. This means that stable atoms have a neutral charge.
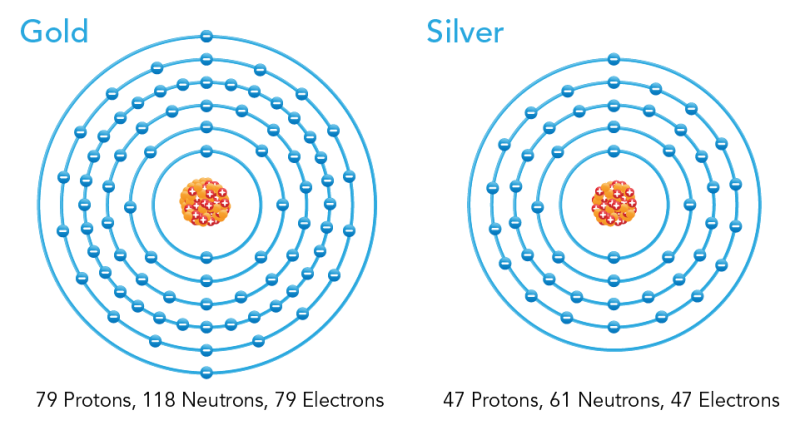
Image - Text Version
Shown is a colour diagram of two different atoms with neutrons shown along concentric rings around a nucleus at the centre. The atom on the left is labelled "Gold." This has a clump of orange spheres and red spheres with plus signs at the centre. Blue spheres with minus signs are neatly arranged around six concentric blue circles around it. Text below the diagram reads "79 Protons, 118 Neutrons, 79 Electrons." The atom on the right is labelled "Silver." This has a clump of orange spheres and red spheres with plus signs at the centre. Around this, fewer blue spheres with minus signs are neatly arranged along five concentric blue circles. The text below the diagram reads "47 Protons, 61 Neutrons, 47 Electrons."
Did you know?
Any particle smaller than an atom is called a subatomic particle. Protons, neutrons and electrons are all subatomic particles.
Atomic Models
It can be tough for people to understand things they cannot see. This was the case for the atom. Over time, scientists created different scientific models. They did this as they performed experiments and made observations. These models helped them explain and predict how atoms behave.
Thomson Plum Pudding Model
At the start of the 20th century, J.J. Thomson did experiments to learn about the atom. He showed that positively-charged and negatively-charged particles made up atoms. What he was not sure about was how they fit together. His idea at the time was that the negative electrons were stuck into a positive sphere. He imagined that the atom looked like a popular Christmas cake that had raisins in it. That is why this model is known as the plum pudding model.
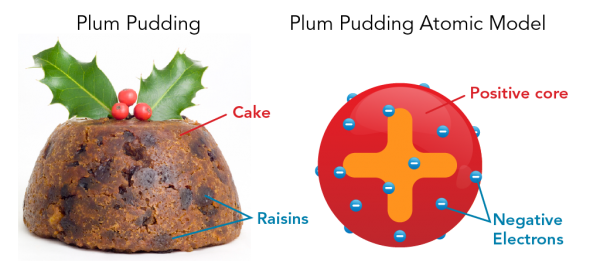
Image - Text Version
Shown are a colour photograph and a colour diagram illustrating the plum pudding atomic model. On the left, the photograph is labelled "Plum pudding." It shows a golden brown, dome-shaped cake with dark brown, oval shapes throughout. The golden brown area is labelled "Cake", and the dark ovals are labelled "Raisins." On top is a sprig of holly with two green leaves and three red berries. The diagram is labelled "Plum Pudding Atomic Model." It shows a red sphere with a large orange plus sign in the centre. Small blue spheres with white minus signs are stuck in the red sphere, similar to the raisins in the pudding. The red area is labelled "Positive core" and the blue spheres are labelled "Negative Electrons."
Rutherford Planetary Model
Scientists’ understanding of the atom changed in 1911. This was due to the gold foil experiment done by Ernest Rutherford and his team. In their work, they saw that the positive charge of atoms seemed to be concentrated at their centres. Rutherford called this the nucleus. He also predicted that the electrons would orbit the nucleus, like planets around the Sun. This is why Rutherford's model is also called the planetary model. You have probably seen this model. Lithium atoms, like the one below, are often used as a symbol to represent science!
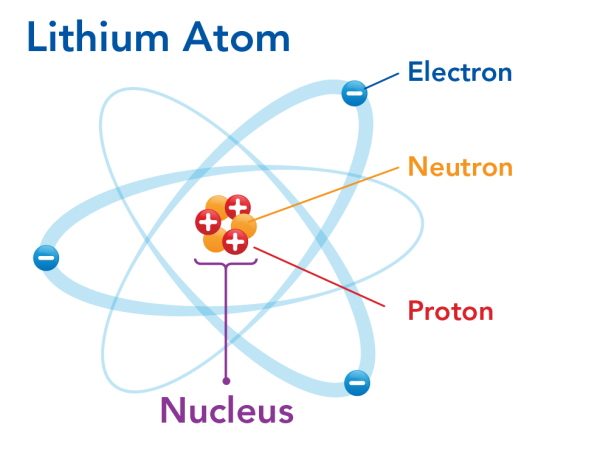
Image - Text Version
Shown is a colour diagram of an atom showing electrons moving around a nucleus. The diagram is titled "Lithium Atom." In the centre is a clump of three red spheres with plus signs and three orange spheres. One red sphere is labelled "Proton: Positively charged particles." One orange sphere is labelled " Neutron: Particles with no charge." The whole clump is labelled "Nucleus." Around the nucleus are three blue spheres with minus signs. The top one is labelled "Electron: Negatively charged particles." Each electron orbits the nucleus on a separate, pale blue oval path. These are fanned out evenly around the nucleus in the centre.
Bohr Model
Rutherford’s planetary model explained a lot. But it didn’t answer some questions that scientists still had. They wondered where the electrons actually were. Could their location be predicted? They also wondered why the orbiting electrons didn’t lose energy and crash into the nucleus. Luckily, Danish scientist Niels Bohr was trying to find those answers.
Bohr was part of a group of scientists interested in a new field called quantum mechanics. Quantum mechanics is the study of how atomic particles exist and interact with each other. Bohr was particularly interested in the energy possessed by electrons.
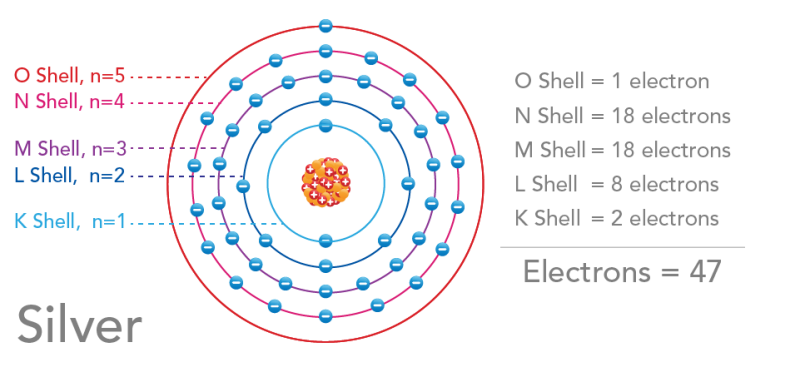
Scientists were beginning to understand more about energy and subatomic particles. Based on this, Bohr suggested that electrons orbit the nucleus along specific paths. He called these paths electron shells. Remember the atomic models of gold and silver above? Those are Bohr models. The electron shells are labelled using either letters (K, L, M, N, O, P, Q) or quantum numbers (n=1 to n=7).
Image - Text Version
Shown is a colour diagram of a silver atom with electron shells in different colours, labelled with letters and quantum numbers. The title "Silver" is in the lower left corner. In the centre, a nucleus is illustrated with a clump of orange spheres and red spheres with white minus signs. Around this are five concentric circles of coloured lines. Starting closest to the nucleus, the first circle is pale blue. This is labelled "K shell, n=1." The next circle is dark blue, labelled "L shell, n=2." The next is purple, labelled "M shell, n=3." The next is pink, labelled "N shell, n=4." The circle on the outside is red and labelled "O shell, n=5." On the right is a list of the shells with the number of electrons in each. This reads, "O shell = 1 electron, N shell = 18 electrons, M shell = 18 electrons, L shell = 8 electrons, K shell = 2 electrons." Under a horizontal line at the bottom, the total is in bold letters, "Electrons = 47."
Electron Cloud Model
The Bohr Model quickly became popular. We still use it today because it helps us understand how and why atoms interact with each other. But scientists were not finished trying to understand how atoms look.
In 1926, Austrian physicist Erwin Schrödinger took Bohr’s model a step further. He proposed a model which described the likelihood of finding an electron in a given place. This model is known as the electron cloud model or the quantum mechanical model. Drawings of electron clouds look like fuzzy shapes. Where the shape is the most dense, there is the most chance of finding an electron. The area where an electron is most likely to be found is called its orbital.
Image - Text Version
Shown is a black and white diagram of an electron made up of hundreds of tiny black dots. The diagram looks like pepper, or another black powder, spilled on a table, but pushed into specific shapes. In the centre is a small, thick dark ring like a donut. The black dots are concentrated along the ring shape, where they appear thick and dark. But the edges are fuzzy with scattered dots. This is labelled "Nucleus." A much larger, thinner, fuzzier ring is centred around the first. Here, the dots are less dense, so you can see each one individually. They are concentrated along the shape of the ring, but scattered widely and thinly around the edges. This is labelled "Electron "cloud" = electron orbital."
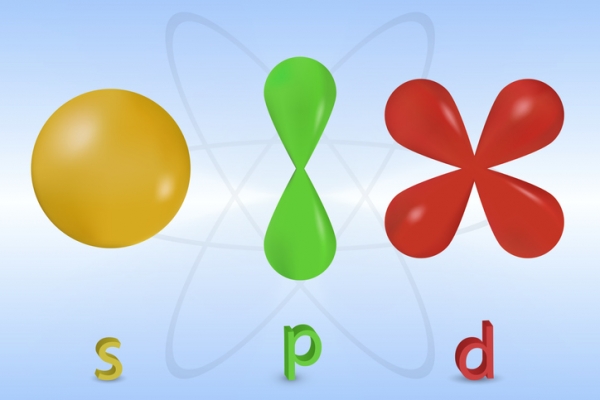
Remember that the Bohr model showed the location of electrons in shells? That is still important in this model. Within each shell are subshells. Within each subshell are a specific number of orbitals. Each of the subshells can hold a certain number of electrons. They also have characteristic shapes. These shapes can get pretty complex as the number of electrons increases. Below are the shapes of the s, p and d subshells.
Image - Text Version
Shown is a colour diagram of three different smooth, rounded shapes, similar to balloons. The first shape is a yellow sphere. This is labelled "s." The second is green. It is two teardrop shapes arranged vertically. The upper is upside-down and the lower is right side up and they are joined together at the pointed ends. This is labelled "p." The third is red. It has four teardrop shapes. They are arranged in an X shape so the pointed ends join in the centre. This is labelled "d."
Atomic models are a great example of how scientific thinking changes over time. As well as how new tools, such as computer modeling, can lead to new ways of things about how things work.
Learn More
The Atom
This resource from Edumedia has a brief history of the atom and includes animations of different atomic models.
This is Not What an Atom Looks Like (2017)
This video from SciShow (5:09 min.) explores the models that have been used to describe atoms.
Introduction to Quantum Mechanics (2020)
This backgrounder from Let’s Talk Science describes the history of quantum mechanics and how we use these ideas every day.
Orbitals: Crash Course Chemistry #25 (2013)
This video from CrashCourse (10:51 min.) discusses what molecules actually look like and why, and explores some quantum-mechanical three-dimensional wave functions.
References
Brittanica. (n.d.). Orbital.
cK-12. (2018). 3.17 Electron Cloud Atomic Model.
Compound Chemistry. (2016, Nov. 16). The History of the Atom.
Crash Course. (2013). Orbitals: Crash Course Chemistry #25.
Deziel, C. (2018). What Is an Unstable Atom? Sciencing.com.
Helmenstine, A.M. (2019, May 6). Basic Model of the Atom and Atomic Theory. Thought Co.
Khan Academy. (n.d.). The quantum mechanical model of the atom.
Khan Academy. (n.d.). Bohr’s model of hydrogen.
Lumen. (n.d.). Reading: Electrons.
Paterson, D. (2019, March 26). States of matter and particle theory. Education in Chemistry.
Scribblegoose. (2013). Quantum Mechanics: Schrödinger's discovery of the shape of atoms.
State Government of Victoria, Australia. (2020). Scientific Models.
Study.com. (n.d.). Modern Atomic Theory: Electron Clouds, Schrodinger & Heisenberg. Holt Physics online.
TechnologyUK. (n.d.). Electron Shells and Orbitals.
Williams, M. (2016. April 8). What Is The Electron Cloud Model? Universe Today.