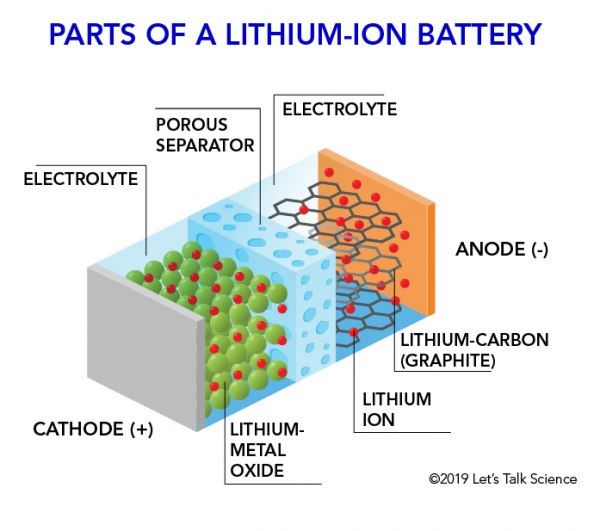
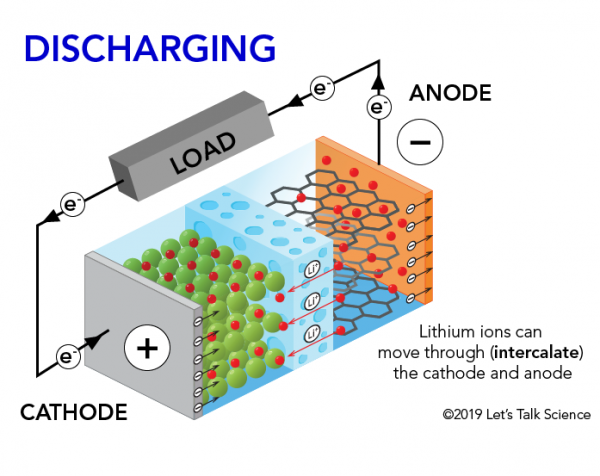
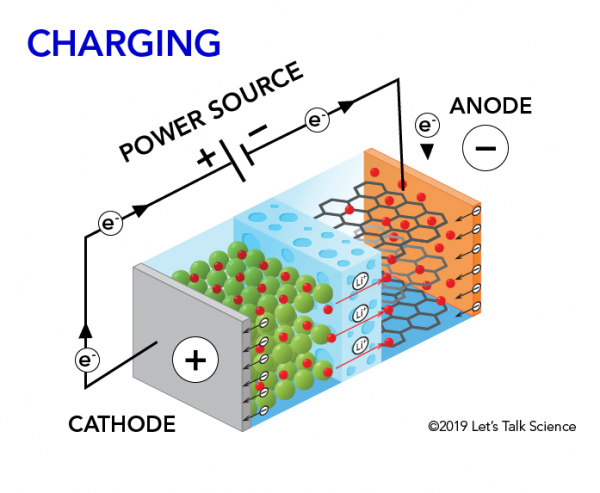
How does a lithium-Ion battery work?

3.7 V lithium-ion battery (AlexLMX, iStockphoto)

3.7 V lithium-ion battery (AlexLMX, iStockphoto)
8.16
How does this align with my curriculum?
Curriculum Alignment
YT
12
Chemistry 12 (British Columbia, June 2018)
Big Idea: Oxidation and reduction are complementary processes that involve the gain or loss of electrons.
AB
9
Knowledge and Employability Science 8, 9 (revised 2009)
Unit D: Electrical Principles and Technologies
NU
9
Knowledge and Employability Science 9 (Alberta, Revised 2009)
Unit D: Electrical Principles and Technologies