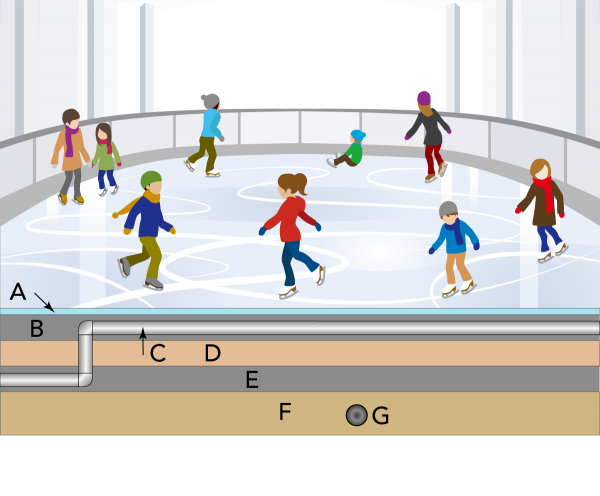
Keeping the Rink on Ice

General Motors Place, Vancouver, British Columbia (Mister Leung [CC BY-SA 2.0], Wikimedia Commons)

General Motors Place, Vancouver, British Columbia (Mister Leung [CC BY-SA 2.0], Wikimedia Commons)
6.28
How does this align with my curriculum?
Curriculum Alignment
AB
6
Science 6 (2023)
Matter: Understandings of the physical world are deepened by investigating matter and energy.
AB
3
Science 3 (2023)
Matter: Understandings of the physical world are deepened by investigating matter and energy.
BC
8
Science Grade 8 (June 2016)
Big Idea: The behaviour of matter can be explained by the kinetic molecular theory and atomic theory.