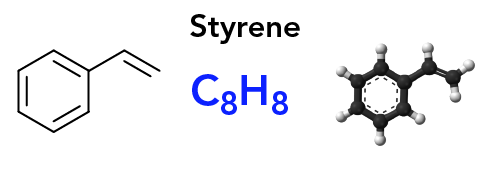
Polystyrene: The Pros, the Cons, the Chemistry

plastic food containers, trays and food packaging made from polystyrene (smartboy10, iStockPhoto)

plastic food containers, trays and food packaging made from polystyrene (smartboy10, iStockPhoto)
7.09
How does this align with my curriculum?
Curriculum Alignment
BC
11
Chemistry 11 (June 2018)
Big Idea: Organic chemistry and its applications have significant implications for human health, society, and the environment.
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Organic chemistry and its applications have significant implications for human health, society, and the environment.
NU
11
Chemistry 20 (Alberta, 2007, Updated 2014)
Unit A: The Diversity of Matter and Chemical Bonding
NT
11
Chemistry 20 (Alberta, 2007, Updated 2014)
Unit A: The Diversity of Matter and Chemical Bonding
ON
11
Environmental Science, Grade 11, University/College (SVN3M)
Strand E: Reducing and Managing Waste
AB
4
Science 4 (2023)
Matter: Understandings of the physical world are deepened by investigating matter and energy.