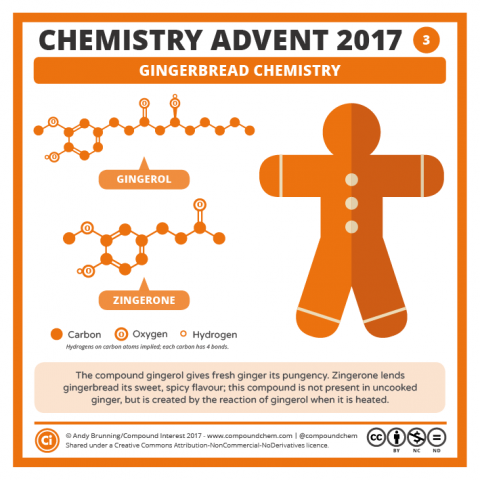
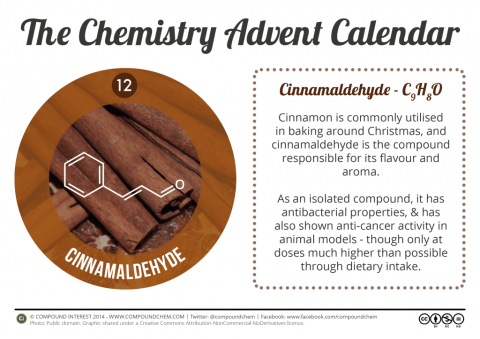
A Merry Molecular Christmas

Image © istockphoto.com/ariwasabi

Image © istockphoto.com/ariwasabi
8.6
How does this align with my curriculum?
Curriculum Alignment
BC
11
Chemistry 11 (June 2018)
Big Idea: Organic chemistry and its applications have significant implications for human health, society, and the environment.