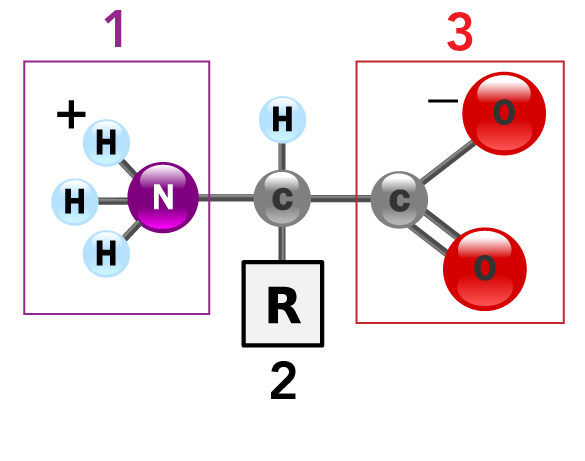
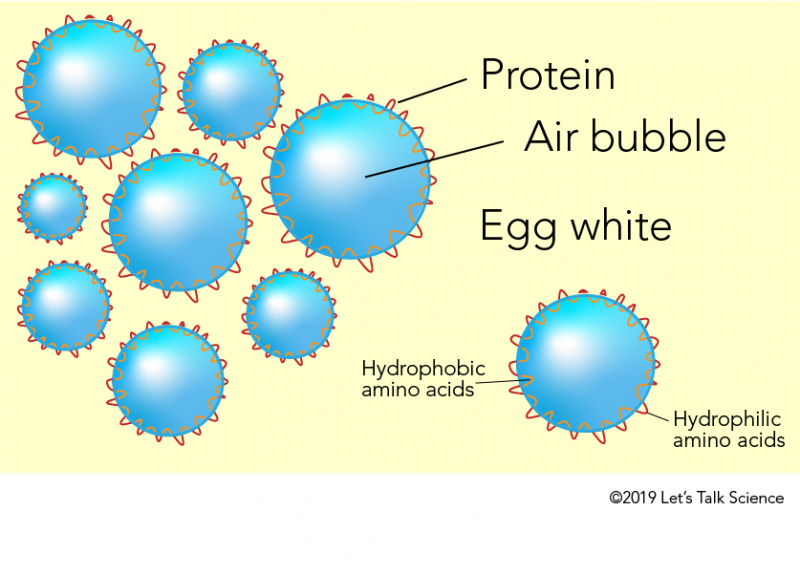
Meringue: The Science Behind a Wonderfully Fluffy Dessert

Pavlova meringue with fruit (KateSmirnova, iStockphoto)

Pavlova meringue with fruit (KateSmirnova, iStockphoto)
6.88
How does this align with my curriculum?
Curriculum Alignment