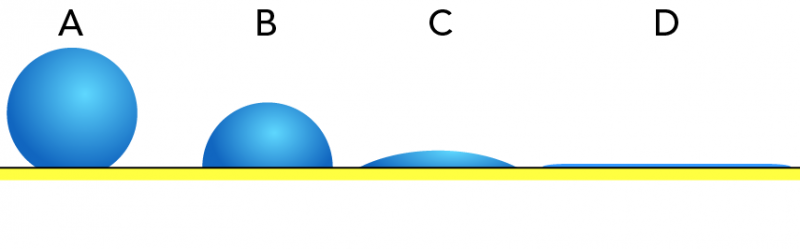
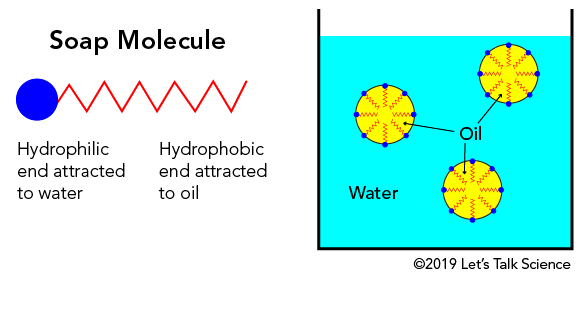
Stupendous Suds

A person washes hands with soap (jacqueline macou, Pixabay)

A person washes hands with soap (jacqueline macou, Pixabay)
7.07
How does this align with my curriculum?
Curriculum Alignment
BC
11
Chemistry 11 (June 2018)
Big Idea: Organic chemistry and its applications have significant implications for human health, society, and the environment.
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Organic chemistry and its applications have significant implications for human health, society, and the environment.
BC
10
Science Grade 10 (March 2018)
Big Idea: Energy change is required as atoms rearrange in chemical processes.
NU
10
Science Grade 10 (British Columbia, June 2016)
Big Idea: Energy change is required as atoms rearrange in chemical processes.
ON
10
Science Grade 10 Applied (SNC2P)
Strand C: Chemical Reactions and Their Practical Applications
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Matter and energy are conserved in chemical reactions.