Why Don't Fish Freeze in Cold Arctic Waters?

Arctic pack ice (KenCanning, iStockphoto)

Arctic pack ice (KenCanning, iStockphoto)
How does this align with my curriculum?
| Grade | Course | Topic |
|---|
Ever wonder how fish survive in the cold waters of the Arctic and Antarctic? It’s partly because of a brilliant evolutionary adaptation.
If you live in Canada, winter eventually “gets in your veins,” so to speak. But the animal kingdom has one-upped people in this department. There are actually fish that live in the Arctic and Antarctic that have antifreeze running through their veins!
Antifreeze is a substance that keeps a liquid from freezing. It lowers the freezing temperature, so it prevents damage that could be caused by ice formation. For example, if you’ve ever driven or ridden in a car in winter, antifreeze is what’s keeping the liquid in that car’s radiator from freezing.
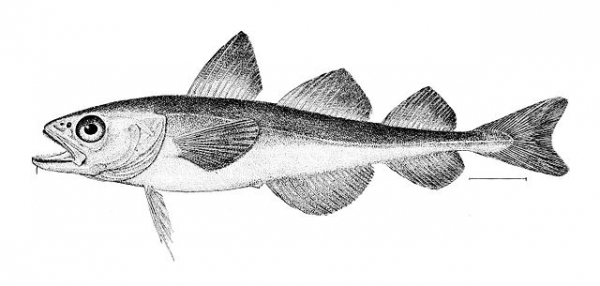
Fish living in cold climates have evolved an adaptation to keep from freezing: antifreeze proteins. Arctic and Antarctic fish families have these proteins in their blood. They’re part of why these fish can live in waters that other fish can’t.
Did you know?
Because of its salt content, seawater freezes at -2°C, not 0°C.
Because fish are ectothermic (cold-blooded) animals, when the water they live in is below freezing, they need a way to keep themselves from freezing. This is where the antifreeze proteins come in.
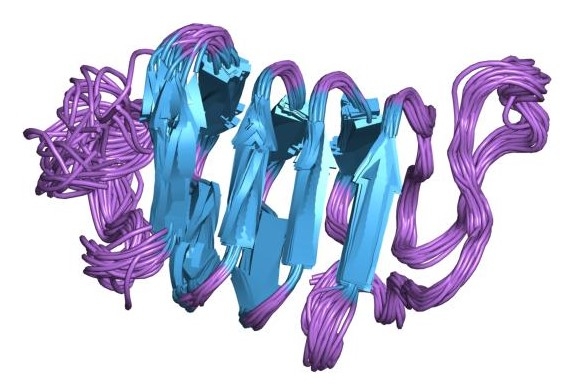
Antifreeze proteins have long strands of repeating amino acid units that can bind to ice crystals.
Ice crystals are dangerous to the fish because their formation in blood leads to cell death. Small ice crystals can grow larger using surrounding water in the blood. Antifreeze proteins can prevent this by binding to the small crystals. This prevents the crystals from growing, and prevents the blood from freezing.
Did you know?
Humans have found ways to use antifreeze proteins, too. For example, ice cream manufacturers use them to prevent ice crystals from forming. Scientists and lab workers also use them when they store tissues or organs.
Some of these antifreeze proteins came to be millions of years ago, and have evolved multiple times. They’ve evolved whenever a type of fish has needed to change in order to survive.
Antifreeze proteins are an example of convergent evolution. This is when organisms of different species with no close relations nonetheless develop similar characteristics. This usually happens because they’re trying to survive in similar environmental conditions and because there may only be a limited number of options to solve a problem they encounter.
For example, the Antarctic toothfish and the Arctic cod have no close relatives. They also live very far away from each other, almost on opposite ends of the Earth! But they have both evolved antifreeze proteins. That’s because they both live in environments where, without these proteins, they’d freeze to death.
What does all this mean? Thanks to antifreeze proteins, fish can live in areas where they otherwise couldn’t. What an amazing evolutionary adaptation!
Starting Points
- What does your community use to make icy sidewalks and roads less slippery - sand or salt? Why do you think this is the case?
- Have you ever gone ice fishing? Why do the fish not freeze under the ice?
- How has knowledge about antifreeze proteins impacted other areas of science?
- Could antifreeze proteins be a more ecologically-friendly alternative to road salt? What barriers might exist to using this technology for road safety?
- What is convergent evolution? Can you think of another example of convergent evolution that is not presented in this article?
- How are proteins formed in a living thing?
- Why does salt water freeze at a lower temperature than freshwater?
- What other adaptations do fish have that live in very cold waters?
- As arctic waters warm up, how might this antifreeze adaptation in arctic fish impact on fish survival?
- In light of what you have learned by reading this article, why is protecting and preserving the existing biodiversity on Earth of value?
- This article supports learning in biology related to adaptations and evolution of organisms. It introduces the concepts of convergent evolution and ectothermic organisms. .
- After reading the article student could also read the Learn More articles to find out more about antifreeze protein adaptations and the animals that have this adaptation..
- Students could complete a Concept Definition Web for the concept of antifreeze proteins or convergent evolution. Download ready-to-use Concept Definition Web reproducibles in [Google doc] or [PDF] formats.
Connecting and Relating
- What does your community use to make icy sidewalks and roads less slippery - sand or salt? Why do you think this is the case?
- Have you ever gone ice fishing? Why do the fish not freeze under the ice?
Relating Science and Technology to Society and the Environment
- How has knowledge about antifreeze proteins impacted other areas of science?
- Could antifreeze proteins be a more ecologically-friendly alternative to road salt? What barriers might exist to using this technology for road safety?
Exploring Concepts
- What is convergent evolution? Can you think of another example of convergent evolution that is not presented in this article?
- How are proteins formed in a living thing?
- Why does salt water freeze at a lower temperature than freshwater?
- What other adaptations do fish have that live in very cold waters?
- As arctic waters warm up, how might this antifreeze adaptation in arctic fish impact on fish survival?
Nature of Science/Nature of Technology
- In light of what you have learned by reading this article, why is protecting and preserving the existing biodiversity on Earth of value?
Teaching Suggestions
- This article supports learning in biology related to adaptations and evolution of organisms. It introduces the concepts of convergent evolution and ectothermic organisms. .
- After reading the article student could also read the Learn More articles to find out more about antifreeze protein adaptations and the animals that have this adaptation..
- Students could complete a Concept Definition Web for the concept of antifreeze proteins or convergent evolution. Download ready-to-use Concept Definition Web reproducibles in [Google doc] or [PDF] formats.
Learn more
Antifreeze Proteins (2009)
More detailed information and some attractive pictures including a rotatable interactive image from PDB-101.
Article from Understanding Evolution on how fish are able to survive in arctic waters without their blood freezing.
References
Committee on Fundamental Science. (1996). United States Antarctic program. National Science and Technology Council.