Catalytic Converters

Platinum crystals (Jurii, Wikimedia Commons)

Platinum crystals (Jurii, Wikimedia Commons)
How does this align with my curriculum?
Learn about how catalytic converters make vehicle emissions less harmful.
What comes out of a car’s exhaust pipe?
If a vehicle burns fossil fuels, then nasty gases come out of its tailpipe. A more fancy term for these gases are emissions. Vehicle emissions contain many different chemical compounds. Some are more harmful than others.
Most vehicle engines use gasoline for fuel. A vehicle mixes this fuel with air before burning it. This chemical process is called combustion. When gasoline burns in air, it produces many chemical by-products.
Some of these by-products are perfectly safe. For example, air is 78% nitrogen gas (N2). Some of this nitrogen reacts with oxygen during combustion. This produces some nitrogen oxides (NOx), which are toxic. However, most nitrogen gets released as N2.
Engine exhaust also includes water (H2O). You’ll often see water dripping from exhaust pipes during the winter.
Some byproducts of combustion can cause health problems. These include breathing difficulties, cardiovascular disease and cancer. They are caused by nitrogen oxides (NOx), unburned hydrocarbons, carbon particles, and volatile organic compounds (VOCs).
Some byproducts can also pollute our environment. Acid precipitation, air and water pollution are caused by carbon dioxide (CO2), nitrogen oxides (NOx) and sulfur oxides.
Car engines also release carbon monoxide (CO). This poisonous gas can replace oxygen in your bloodstream. If you breathe enough of it, you could suffocate!
That all sounds very dangerous, doesn’t it? Fortunately, catalytic converters help make engine emissions less harmful. Here’s how.
What is a catalytic converter?
Eugène Houdry invented the catalytic converter around 1950. The French mechanical engineer had spent his career developing better fuels for cars. But by 1950, scientists were beginning to learn about air pollution caused by cars. So, Houdry designed the catalytic converter to clean exhaust.
The leaded gasoline used in 1950 damaged catalytic converters. By 1975, scientists had developed unleaded gasoline. That year, the U.S. Environmental Protection Agency made catalytic converters mandatory on all new cars. Other countries soon followed.
Did you know?
Cars have catalytic converters, but lawn mowers do not. If you burned the same amount of fuel in a car and a lawnmower, the lawnmower would emit about 100 times more pollution!
How do catalytic converters work?
The catalytic converter is attached to the exhaust pipe underneath a car. It is a metal box with a ceramic honeycomb inside. The honeycomb is coated with a mix of platinum (Pt), palladium (Pd) and rhodium (Rh). These noble metals are good at resisting oxidation, corrosion and acid. This means they can stand up to all the chemicals released by a car engine.
Misconception Alert
Noble metals and precious metals aren’t the same thing. Precious metals have a high monetary value. Noble metals are highly resistant to corrosion and oxidation. However, some precious metals are also noble metals.
The metals in catalytic converters are catalysts. Catalysts are compounds that trigger a chemical reaction without being affected themselves. Catalytic converters have a honeycomb structure because it provides a lot of surface area for a lot of reactions.
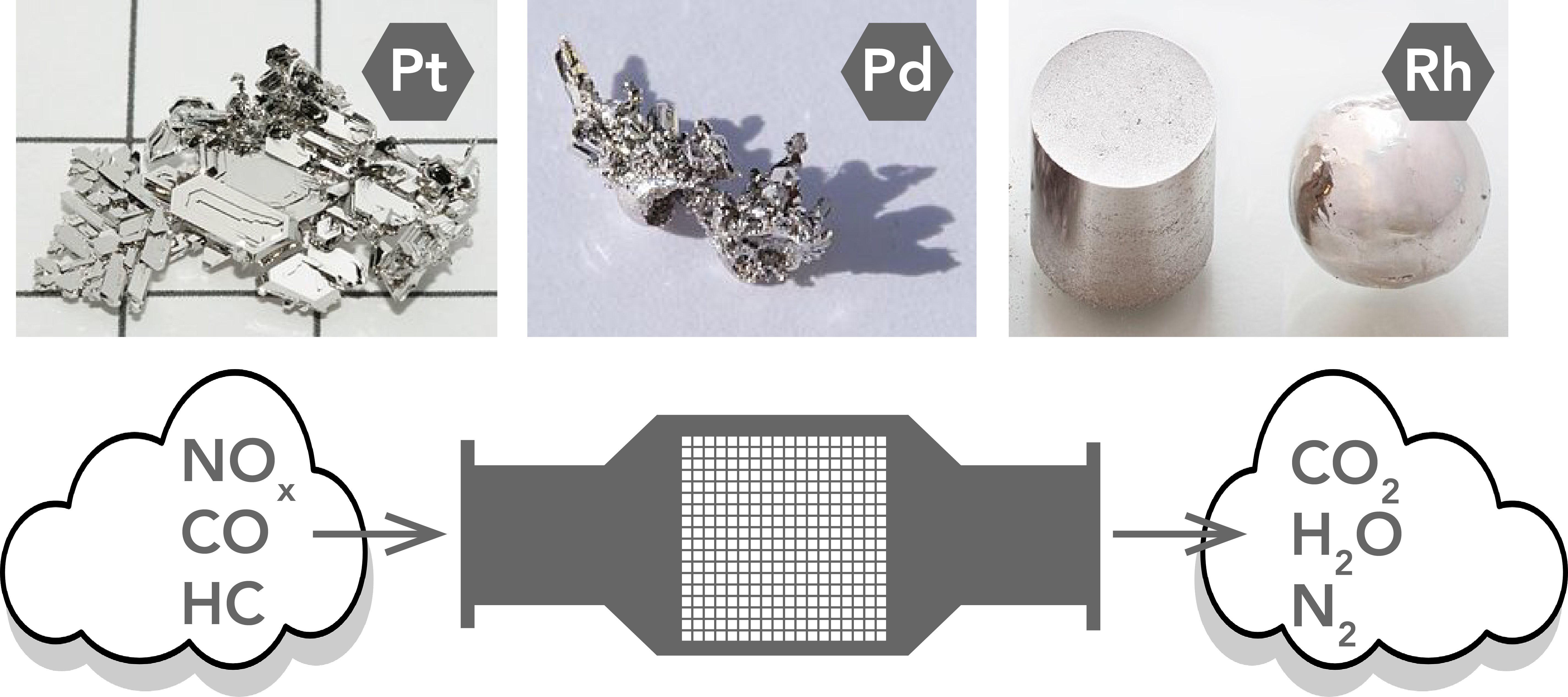
Catalytic converters use elements like Platinum (Pt), Palladium (Pd), and Rhodium (Rh) as catalysts (Let’s Talk Science using photographs by Periodictableru [CC BY], Hi-Res Images of Chemical Elements [CC BY and Alchemist-hp (talk) www.pse-mendelejew. derivative work: Purpy Pupple [CC BY-SA 3.0] Wikimedia Commons (Pt, Pd, Rh)).
Image - Text Version
Shown are three colour photographs of noble metals and a black and white illustration of a catalytic converter.
From left to right, the first photograph shows platinum. It is a shiny, bright silver pile of flat pieces with straight edges.
The next photograph shows palladium. It is a darker, shiny silver, in two chunks that look like crumpled, twisted tinfoil.
The last photograph is rhodium. It is bright, shiny, silver in two shapes. One is a cylinder, and the other is a sphere. Both are smooth and polished.
Below, the illustration shows the process that happens in a catalytic converter. From left to right, it begins with a puffy white cloud that contains the chemical symbols for nitric oxides, carbon monoxide, and hydrocarbon.
An arrow points from here into a grey box with a pipe on either side.There is a grid of tiny white squares inside.
On the right of the box, an arrow points out from the pipe, into another white cloud. This one contains the chemical symbols for carbon dioxide, water, and nitrogen gas.
Did you know?
Today, about 98% of all new vehicles sold worldwide contain a catalytic converter.
What chemical reactions happen in a catalytic converter?
The catalysts in catalytic converters cause oxidation and reduction (redox) reactions. These reduce harmful emissions.
Platinum and rhodium take part in the reduction reactions. These reduce nitrogen oxides (NOx) in exhaust. They do this by removing nitrogen atoms from nitrogen oxide molecules (NO and NO2). This means the free oxygen atoms form oxygen gas (O2).
Then, the nitrogen atoms attached to the catalyst react with each other. This creates nitrogen gas (N2). Oxygen and nitrogen gases are both safe to breathe.
Reduction Reactions
Nitric acid 2NO → N2 + O2
Nitrogen dioxide 2NO2 → N2 + 2O2
Platinum and palladium take part in oxidation reactions. These reduce hydrocarbons (HC) and carbon monoxide (CO) in exhaust. First, carbon monoxide and oxygen combine to form carbon dioxide (CO2). Then, unburned hydrocarbons and oxygen combine to form carbon dioxide and water. Carbon dioxide is safe to breathe at low concentrations.
Oxidation Reactions
Reaction 1: 2CO + O2 → 2CO2
Reaction 2: HC + O2 → CO2 + H2O
Modern catalytic converters also have an oxygen sensor. It detects the ratio of fuel and air in the exhaust. Too much fuel in the engine leaves unburnt hydrocarbons after combustion. Too much oxygen produces more nitrogen oxides. If the ratio is not correct, the oxygen sensor changes the amount of fuel going into the engine.
Did you know?
An engine produces the most pollution right after you start it. That’s because catalytic converters only work at high temperatures. And cars take a few minutes to warm up. This is a great reason to walk or bike short distances!
Rhodium and palladium are some of the most valuable metals in the world. That’s because they are rare, and we need them. People even steal catalytic converters to get at the precious noble metals inside.
Starting Points
- Would you use an engine if you knew it didn’t have a catalytic convertor? Why or why not?
- Do you try to limit the amount of pollution you produce? Explain.
- Have you experienced smog or heavy air pollution? Describe the effects.
- What are some examples of noble metals used as catalysts in catalytic converters? Should governments invest more in the production of these metals? Why or why not?
- Should we continue to use noble metals in catalyst in catalytic converters? Or should we use more environmentally friendly cars? Explain.
- What is a catalyst? What catalysts are commonly used in catalytic converters?
- What role does the catalyst play in reducing harmful emissions from car exhaust? What chemical reactions occur during this process?
- Catalytic converters still allow carbon dioxide to be produced during combustion. Carbon dioxide is a major contributor to climate change. Is the production of nitrogen oxides and sulphur oxides worse than the emission of carbon dioxide for the environment? Explain.
- What is the function of the oxygen sensor in a vehicle’s exhaust system?
- This article can be used to support teaching and learning of Chemistry, Environmental Science, Pollution and Technology & Engineering related to air quality, catalysts, combustion, redox reactions, metals and hydrocarbons. Concepts introduced include gasoline, hydrocarbon, combusting, carbon dioxide, nitrogen oxides (NOx), sulfur oxides, acid precipitation, volatile organic compounds (VOCs), carbon monoxide, catalytic converter, catalysts, compounds, oxidation, reduction, reduction catalyst, Nitric acid, Nitrogen dioxide, oxidative catalyst and oxygen sensor.
- After reading the article, teachers could have students complete a Key Ideas Round Robin learning strategy to summarize the key ideas from the article. Ready-to-use Key Ideas Round Robin reproducibles are available in [Google doc] and [PDF] formats.
- For teaching about catalysts in Chemistry, teachers could use this article as an example of an application of catalysts that are used in everyday life.
- To go further, teachers could have students debate the use of gold as a catalyst in catalytic converters using a Pros & Cons Organizer learning strategy. Ready-to-use Pros & Cons reproducibles in [Google doc] and [PDF] formats.
- To have students investigate the issues surrounding transportation and fossil fuels and learn about the carbon footprint associated with different modes of transportation in society, teachers could have students participate in the Energy4Travel Action Project.
Connecting and Relating
- Would you use an engine if you knew it didn’t have a catalytic convertor? Why or why not?
- Do you try to limit the amount of pollution you produce? Explain.
- Have you experienced smog or heavy air pollution? Describe the effects.
Relating Science and Technology to Society and the Environment
- What are some examples of noble metals used as catalysts in catalytic converters? Should governments invest more in the production of these metals? Why or why not?
- Should we continue to use noble metals in catalyst in catalytic converters? Or should we use more environmentally friendly cars? Explain.
Exploring Concepts
- What is a catalyst? What catalysts are commonly used in catalytic converters?
- What role does the catalyst play in reducing harmful emissions from car exhaust? What chemical reactions occur during this process?
- Catalytic converters still allow carbon dioxide to be produced during combustion. Carbon dioxide is a major contributor to climate change. Is the production of nitrogen oxides and sulphur oxides worse than the emission of carbon dioxide for the environment? Explain.
- What is the function of the oxygen sensor in a vehicle’s exhaust system?
Teaching Suggestions:
- This article can be used to support teaching and learning of Chemistry, Environmental Science, Pollution and Technology & Engineering related to air quality, catalysts, combustion, redox reactions, metals and hydrocarbons. Concepts introduced include gasoline, hydrocarbon, combusting, carbon dioxide, nitrogen oxides (NOx), sulfur oxides, acid precipitation, volatile organic compounds (VOCs), carbon monoxide, catalytic converter, catalysts, compounds, oxidation, reduction, reduction catalyst, Nitric acid, Nitrogen dioxide, oxidative catalyst and oxygen sensor.
- After reading the article, teachers could have students complete a Key Ideas Round Robin learning strategy to summarize the key ideas from the article. Ready-to-use Key Ideas Round Robin reproducibles are available in [Google doc] and [PDF] formats.
- For teaching about catalysts in Chemistry, teachers could use this article as an example of an application of catalysts that are used in everyday life.
- To go further, teachers could have students debate the use of gold as a catalyst in catalytic converters using a Pros & Cons Organizer learning strategy. Ready-to-use Pros & Cons reproducibles in [Google doc] and [PDF] formats.
- To have students investigate the issues surrounding transportation and fossil fuels and learn about the carbon footprint associated with different modes of transportation in society, teachers could have students participate in the Energy4Travel Action Project.
Learn more
Catalytic Converters are getting stolen all across the country. Here's why. (2022)
This video (5:36 min.) by Scientific American, explains why rhodium is one of the most expensive metals on the planet.
Vehicle Emissions Primer (2019)
This backgrounder by Let’s Talk Science explains how an internal combustion engine works, how exhaust contributes to smog, and its consequences.
Oxidation–reduction (redox) reactions
This article by Khan Academy explains oxidation-reduction, or redox reactions, with examples and practice equations.
This metal is more valuable than gold (2021)
This video (6:59 min.) by The Verge, explains how palladium is mined, or recycled, and it is used.
References
Babad, M. (Mar. 9, 1985). Canada to require catalytic converters on U.S. auto imports. UPI Archives.
Government of Canada. Causes of climate change. Canada.ca
Helmenstine, A. M. (2018, September 19). Noble metals list and properties. ThougthCo.
International Platinum Group Metals Association. (n.d.). Catalytic converters.
Mast, L. (2018, December 27). Shortages of rare earth elements could limit clean energy development. Massive.
Nice, K., & Bryant, C. W. (2000, July 11). How catalytic converters work. How Stuff Works.
Roberts, J. (Jan. 12, 2015). Clean Machine. Science History Institute.
United States Environmental Protection Agency. Basic Information about NO2.