How Vehicles Pollute the Air

Cars driving through thick smog (plherrera, iStockphoto)

Cars driving through thick smog (plherrera, iStockphoto)
How does this align with my curriculum?
Learn how vehicles affect air quality, including their contribution to smog.
Vehicles are pretty useful. They get people from point A to point B. They also carry lots of stuff. They can get you further and faster than your own two feet can. But they are also quite good at things that aren’t so useful. They are good at contributing to both smog and climate change.
If you've ever been stuck in traffic behind a big heavy truck, you may have smelled something nasty coming from it. But even when you can't smell anything, vehicles emit all sorts of gases. These gases can have negative effects on both people and the planet.
How does an internal combustion engine work?
You probably know that most cars and trucks run on fuel. Some vehicles now also run on electricity. But more often than not, vehicles run on fossil fuels. These include fuels like gasoline and diesel. Fuels like these are burned inside an engine. That's why it’s called an internal combustion engine. Combustion is a type of chemical reaction that gives off heat.
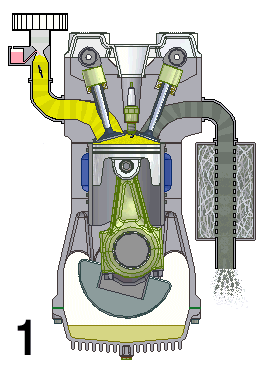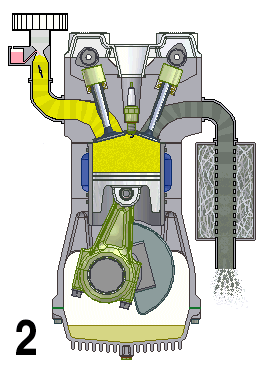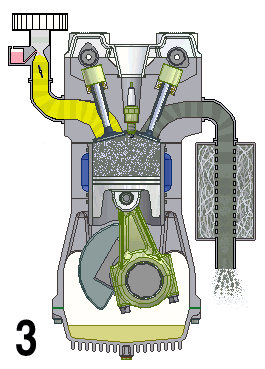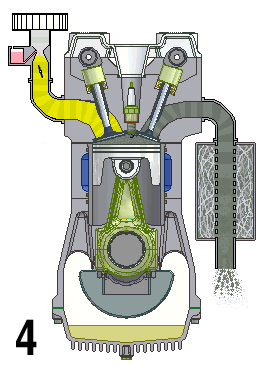
This animation shows the four steps that occur in a combustion engine. The fuel enters at the left and the waste gases exit at the right (Source: UtzOnBike (derivative work) via Wikimedia Commons).
Animation - Text Version
Shown is a colour animated GIF that shows what happens inside an internal combustion engine.
In the bottom left corner, black numbers cycle through 1, 2, 3, and 4 on a loop.
On the top left, a yellow substance flows through a pipe. At the bottom of the pipe, a grey stopper on the end of a moving pole, moves up and down, blocking, then allowing the liquid to move into a chamber below.
When the chamber is full of yellow liquid, a white spark from a green plug in the roof of the chamber turns the chamber red. The chamber then quickly turns grey.
When the chamber is grey, a stopper on the top right of the chamber lets the grey substance up through a pipe. The end of this pipe spews grey fumes, like the exhaust pipe of a car.
At the bottom of the chamber, wide grey object moves up and down in a steady rhythm. It moves down when the chamber becomes yellow, then up until the spark, then down when it's grey.
A large, round green object with a pointed triangular top, hangs from a hinge on the bottom side of the wide grey object. A grey half-circle attached to it moves smoothly around an empty chamber.
After they have pushed the pistons, the gases then leave the engine through the car's tailpipe. We call these gases exhaust. Vehicle exhaust can affect air quality both near the Earth's surface, where we live and breathe, as well as high in the atmosphere. Air quality is a measure of the amount of pollutants in the air.
What is smog?
Smog is a type of short-term air pollution. It occurs in pockets near the ground. You can recognize a very smoggy day by the haze that hangs in the air. Historically, the word "smog" was used to describe the thick, grayish haze that occurred when smoke and fog mixed together. Today, the word smog is used to describe a type of air pollution made up of a mix of different gases and particles in the air.
When there is smog, it can be difficult to see places you might see on a clearer day. You might also find it harder to breathe. The air might also have a bit of a smell. Smog is mainly made up of particulate matter and ground level ozone (O3).
How do vehicles contribute to smog?

A Person holds their shirt over their nose to block out smog (Source: Werawad Ruangjaroon via iStockphoto).
Image - Text Version
Shown is a colour photograph of a person covering their nose and mouth with their t-shirt.
The person is standing on the curb, next to a road full of cars. The air around looks hazy. The person is wearing a black t-shirt, and stretching the fabric up to their face with one hand.
Particulate matter mainly comes from incomplete combustion in car engines. This happens when not all of the fuel is burnt. Incomplete combustion can also happen when burning coal and wood.
Incomplete combustion releases carbon monoxide (CO) as well as unburned fuel, which can become part of smog.
For example, the balanced chemical equation for the complete combustion of methane (CH4) gives us:
CH4 + 2O2 → CO2 + 2H2O
In this reaction, the only products are carbon dioxide (CO2) and water (H2O)
On the other hand, the balanced chemical reaction for the incomplete combustion of methane (CH4) gives us:
4CH4 + 5O2 → 2CO + 8H2O + 2C
In this reaction, the products are carbon monoxide (CO), water and carbon (C).
So far, you’ve read about the direct products of combustion. But there are also indirect products of combustion.
Indirect products of combustion happen when nitrogen and oxygen in the air combine because of the heat released during combustion. These products include nitrogen monoxide (NO) and nitrogen dioxide (NO2). Both of these products are part of a group we call nitrous oxides (NOx). Nitrogen oxides are molecules that only contain nitrogen and oxygen.
Did you know?
Nitrous oxide (N2O) is a colourless gas that is commonly used by dentists for sedation and pain relief. It is also known as “laughing gas.”
Other indirect products of combustion include volatile organic compounds (VOCs), such as benzene and formaldehyde. These compounds are found naturally in crude oil and gasoline. Sulfur dioxide (SO2), which comes from the combustion of diesel fuel, is also an indirect product.
What is smog?
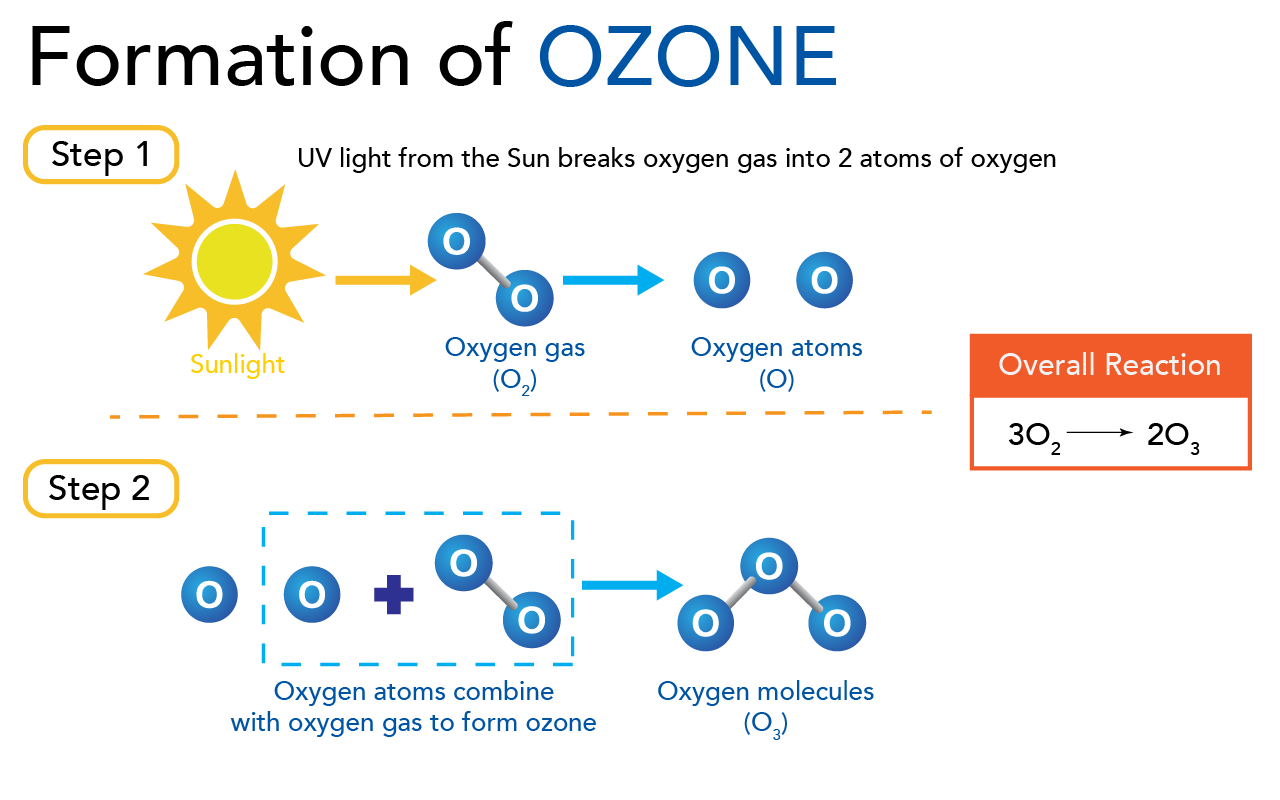
Formation of ozone (Let’s Talk Science using an image by Trinset via iStockphoto)
Image - Text Version
Shown is a colour diagram of the process that forms ozone.
The top part of the image is labelled "Step 1." Below is a picture of the sun, labelled "Sunlight." An orange arrow points from here to the right. Here, two blue circles labelled with the letter O are joined with a grey line. This is labelled "Oxygen Gas (O2)." A blue arrow points from here to the right again. Here, the two blue circles have been separated. This is labelled "Oxygen atoms (O)."
The bottom part of the image is labelled "Step 2." Below, a single oxygen atom is alone on the left. To the right, a dashed blue line is drawn in a rectangle around another oxygen atom, a plus sign, and two joined oxygen atoms. This is labelled "Oxygen atoms combine with oxygen gas to form ozone." A blue arrow points from here, to the right. Here, the last illustration is three oxygen atoms joined together by two grey lines. This is labelled "Oxygen molecules (O2)."
On the right side, a red rectangle is labelled "Overall Reaction." Below, it reads "3O2 --> 2O3."
Ground level ozone is not 'good' ozone. The ozone at ground level is formed when NOx and VOCs react together on still, sunny days. This is why we tend to have smog on hot summer days. But smog can also occur in winter, especially when there are a lot of vehicles on the road.
What are the consequences of smog?

Smog can make it difficult to breathe. In some cities, people even wear masks when outside (Source: ilyaliren via iStockphoto).
Image - Text Version
Shown is a colour illustration of three adults and two children wearing face masks in a smoggy, grey city.
All the people look worried. They are holding their hands near their faces as if they are coughing or having difficulty breathing. One child is holding an adult's hand. Another adult is reaching for the hand of the child next to them.
The background is all grey and beige. The outline of a row of cars is visible behind the people. Behind that are the outlines of skyscrapers. Above, the sky is grey with beige clouds.
The environment is also impacted by smog. When NOx and SO2 react with water in the atmosphere, they form acid precipitation. This can take the form of rain and snow. Acid precipitation can affect the productivity of plants. It can also cause lakes and rivers to become acidic. This can kill or prevent the reproduction of some species. This, in turn, affects whole ecosystems.
Learn More
The Science of Smog (2017)
This TED-Ed video video (5:43) talks about the factors that cause smog and the changes that may help prevent it.
Smog and Your Health (2018)
This article from Health Canada explains the effects of smog on human health, and what you can do to reduce the risks.
What is Acid Rain? (2018)
This video from National Geographic (1:58 min.) explains the causes and effects of acid rain.
References
Brain, M., & Geisler, K. H. (2018, August 16). How car engines work. HowStuffWorks.
Environment & Climate Change Canada. (2013, July 17). Particulate matter.
Government of Canada. (2016, May 19). Common air pollutants: Ground-level ozone.
Government of Canada. (2018, January 31). Smog and your health.
NOAA. (2018, August 1). Climate change: Atmospheric carbon dioxide.