Chemical Equations

Hand writing chemical equations (Igor Kutyaev, iStockphoto)

Hand writing chemical equations (Igor Kutyaev, iStockphoto)
How does this align with my curriculum?
Learn about how to write and balance chemical equations.
You have probably heard the word equation before. It was likely in math class rather than in science, though. An equation is a mathematical statement with an equal sign in it. It means that what is on the left side of the equal sign is equal to what is on the right side of the “=”.
A chemical equation is a way to represent a chemical reaction. The reactants are given on the left hand side and the products are given on the right hand side. Instead of an equal sign, an arrow is drawn between the reactants and the products. It is important to note that the arrow does not mean the same thing as an equal sign. The arrow means that the reactants get converted into products. The general form of a chemical equation looks like:

Image - Text Version
Shown is a general chemical formula using words. On the left is the word "reactants" and on the right is the word "products". Between the two words is an arrow pointing from left to right. Below the arros is the word "form". Considering this as a sentence, you would have "reactants form products".
A chemical equation contains important information about the reaction. The first information is the chemical formula of each product and reactant. A chemical formula represents the number of the different elements that make up a molecule. You might be familiar with some of these popular chemical formulas:
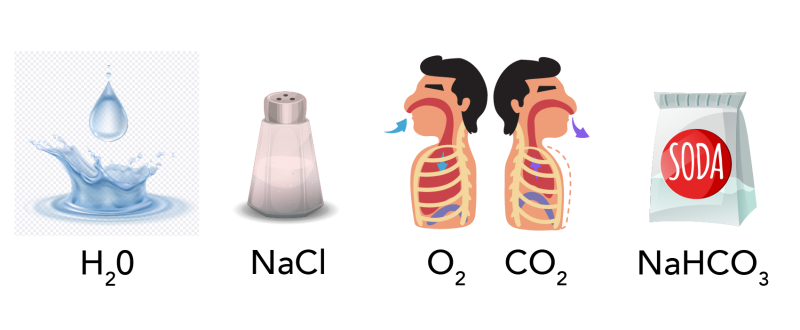
Image - Text Version
Shown are four illustrations and chemical formulas for some common molecules.
On the far left is an image of a water droplet about to hit the surface of water and a splash on the surface of water. Below this is the formula H2O.
To the right of this is an illustration of a salt shaker that is half full of salt. Below this is the formula NaCl.
To the right of this, are two cross sections of a person from the chest up. Visible are the airways, lungs and ribs. In the left image, an arrow points towards the mouth indicating that the person is inhaling. Below this image is the formula O2. By the right image, an arrow points away from the mouth indicating that the person is exhaling. Below this is the formula CO2.
The far right image is of a white paper bag. It has a large, circular label with the text "soda" to indicate that it is a bag of baking soda. Below this is the formula NaHCO3.
Did you know?
The periodic table lists all existent elements. But in nature, those elements rarely exist as single atoms. For instance, oxygen molecules tend to pair up in the air. This is why atmospheric oxygen has a chemical formula of O2. It represents two oxygen bonded atoms.
Skeleton Equations
A skeleton equation is the simple form of a chemical reaction. It includes the chemical formulas, with a “+” between each chemical formula. Here A and B are the chemical formulas of the reactants. C and D are the chemical formulas of the products.
The general form of a skeletal equation is:
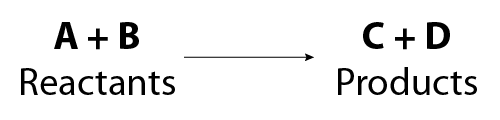
Image - Text Version
Shown is the general form for writing a skeletal equation.
On the left is the text "A + B". Below this is the "Reactants". On the right is the text "C + D". Below this is the word "Products". Between the two sides is an arrow pointing from left to right. This arrow indicates that the reaction proceeds to the right.
Skeleton equations may also give phase tags. These tags show in what phase, also known as a state, a given chemical is in. Those phases can be solid (s), liquid (l), gas (g) and aqueous (aq). Aqueous is a state in which something is dissolved in water.
Below is an example using the classic baking soda and vinegar (acetic acid) reaction. The reactants are solid baking soda and aqueous acetic acid. The products are carbon dioxide gas, liquid water and dissolved sodium (Na+) and acetate (CH3COO-) ions.

Image - Text Version
Shown is a skeletal equation with illustrations for the reaction between solid sodium bicarbonate and an aqueous solution of acetic acid that forms carbon dioxide gas, liquid water, positive sodium ions and negative acetate ions.
The skeletal equation, from left to right, shows that solid NaHCO3 combines with aqueous CH3COOH to form CO2 gas, liquid H2O, Na+ ions and CH3COO- ions.
Below the formula NaHCO3 is an illustration of a bag with the label "soda" indicating that it is baking soda. Below the formula CH3COOH is an illustration of a glass bottle filled with a clear liquid. This illustration represents acetic acid. Below the formula CO2 is an illustration of bubbles. These bubbles represent carbon dioxide gas. Below the formulas for H2O, Na+ CH3COO- is an illustration of a glass bowl containing a clear liquid. The liquid represents an aqueous solution containing sodium ions and acetate ions.
Did you know?
Vinegar consists of acetic acid (CH3COOH), water and trace amounts of other chemicals, which may include flavorings.
Reversible Reactions and Dynamic Equilibrium
Generally, chemical reactions convert reactants to products. In some cases, it is possible to reverse a chemical reaction. This is called a reversible reaction. An example of a reversible reaction happens in rechargeable batteries. In regular batteries, once all chemicals in the battery have been used, the batteries are “dead”. Rechargeable batteries use electrical energy to revert the products into the original reactants.

Sometimes a reversible reaction can have both sides of the reaction happen at the same time. In this case, the reaction reaches a point of equilibrium where the rate of the forward reaction is equal to the rate of the reverse reaction. This is called a dynamic equilibrium.
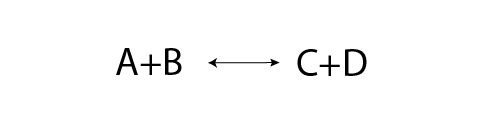
Image - Text Version
Shown is a skeletal equation for a reversible reaction.
On the left is the text "A + B" and on the right is the text "C + D". Between the two sides is an arrow with points at both ends. This arrow indicates that the forward reaction of A + B is going on at the same time as the reverse reaction of C + D.
Sometimes a word or symbol is added above the arrow. This indicates the conditions needed to make a reaction possible. This includes the need for things like an electric current, heat, high pressure, light or a catalyst. Below is an example of an equation that needs heat to happen. The triangle symbol, called delta, indicates that heat is required.
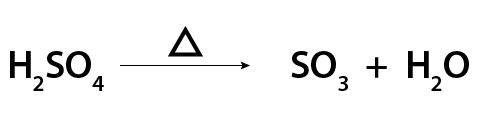
Image - Text Version
Shown is a chemical equation for the breakdown of sulphuric acid in the presence of heat.
On the left side is the equation for the chemical formula for sulphuric acid, H2SO4. On the right side of the equation are the chemical formulas for sulphur trioxide, SO3 and water, H2O. Between the two sides is an arrow pointing from left to right. This arrow indicates that the reaction proceeds to the right. Above the arrow is the symbol of a triangle. This indicates that heat is required for this reaction to occur.
Balanced Chemical Equations
Just like our skeletons form the structure of our bodies, skeleton diagrams form the structure of chemical equations. But there is more to the body than just the skeleton! There is more to chemical equations as well.
The most important information that is missing from a skeleton equation is the amount of reactants and products involved in the reaction. A balanced chemical equation has this additional information. Unlike skeleton equations, balanced equations are adjusted so that the Law of conservation of mass is upheld. This means that mass cannot be created or destroyed. See more about this in the video below.
In a balanced chemical equation we show quantities using coefficients. A coefficient is a number (usually a whole number) that is placed in front of a chemical formula. Coefficients represent the relative ratio with respect to the other chemicals involved in the reaction. The general form of a balanced chemical equation is seen below.

Image - Text Version
Shown is the general form for writing a skeletal equation with coefficients.
On the left is the text "aA + bB". Below this is the word "Reactants". On the right is the text "cC + dD". Below this is the word "Products". Between the two sides is an arrow pointing from left to right. This arrow indicates that the reaction proceeds to the right.
Let’s look at a simple example. Below are the skeleton and balanced equations for the hydrolysis of water. This reaction involves breaking down water molecules using electricity.
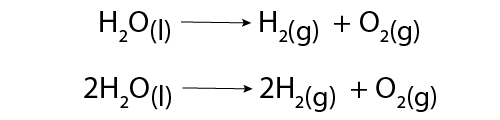
Image - Text Version
Shown are two chemical equations.
In the top equation, one molecule of H2O breaks down to form one molecule of H2 and one molecule of O2. This is indicated with a forward arrow. In this reaction, liquid water forms hydrogen gas and oxygen gas. This is considered to be an unbalanced chemical equation since the number of atoms of each element on each side of the equation are not equal.
In the bottom equation, two molecules of H2O break down to form two molecules of H2 and one molecule of O2. This is indicated with a forward arrow. In this reaction, liquid water forms hydrogen gas and oxygen gas. This is considered to be a balanced chemical equation since the number of atoms of each element on each side of the equation are equal.
You may wonder why two hydrogen molecules (2H2) are needed rather than one. For a balanced chemical equation, the number of atoms on the left side of a chemical equation has to equal the number of atoms on the right side of the equation.
We started off with H2(g) + O2(g) on the right-hand side of the equation. This means that there are two atoms of hydrogen (H2) and two atoms of oxygen (O2):
H2(g) + O2(g)
We know that water is made up of two atoms of hydrogen (H2) and only one atom of oxygen (O):
H2O(l)
This would give us:
H2O(l)→ H2(g) + O2(g)
If we count the atoms on each side, we would see that there are two atoms of hydrogen on the left and two on the right – this is balanced. However, there are two atoms of oxygen on the right but only one on the left. Somehow, we have to make the number of oxygen molecules the same on each side. If we double the number of oxygen atoms on the left side by doubling the number of water molecules, then we would have:
2H2O
The two in front means that if we have two molecules of water, there would be four atoms of hydrogen and two atoms of oxygen.
But now we have four atoms of hydrogen on the left (2H2) but only two on the right (H2)! How do we make both sides equal? Simple – we double the number of H2 on the right which finally gives us:
2H2O(l)→ 2H2(g) + O2(g)
In this reaction, there are four atoms of hydrogen and two atoms of oxygen in both the reactant and the product.
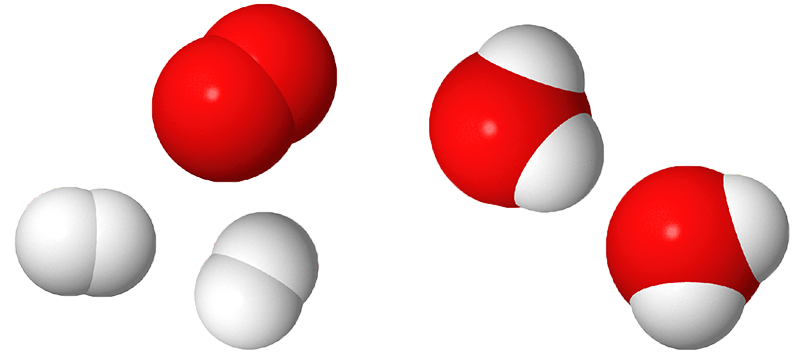
Image - Text Version
Shown are 3D renderings of hydrogen gas, oxygen gas and water molecules. Hydrogen is represented by white, somewhat spherical shapes and oxygen is represented by red, somewhat spherical shapes.
Molecules of hydrogen gas have two of the white shapes stuck together. Two set of these are shown. Molecules of oxygen gas have two of the red shapes stuck together. One set of these is shown. Molecules of water have two of the white shapes stuck to one of the red shapes. Two sets of these are shown.
It is important to remember that the number that is small and below the line refers only to the immediately preceding symbol. A coefficient applies to ALL the following symbols, including the smaller numbers.
It’s your turn!
Question 1: How would you balance the reactants and products in the following reaction?
N2 + O2 → NO
Question 2: How would you balance the reactant and products in the following reaction of hydrogen peroxide (H2O2) into water and oxygen gas?
H2O2 → H2O + O2
Question 3: How would you balance the reactants and products in the following combustion reaction between propane (C3H8), which is used in BBQs, and oxygen (O2)?
C3H8(g) + O2(g) → CO2(g) + H2O(g) + heat and light
Answers
Question 1
How would you balance the reactants and products in the following reaction?
A good way to solve this is to create an inventory of atoms of each element for each side.
| Element | Reactants | Product |
| N | 2 | 1 |
| O | 2 | 1 |
Typically when balancing equations, you start with elements other than oxygen and hydrogen. This is simply because they are usually the most abundant atoms and it is better to balance them at the end. If you double the number of NO in the product, you would get:
| Element | Reactants | Product |
| N | 2 | 2 |
| O | 2 | 2 |
N2 + O2 → 2NO
Now the equation is balanced. There are two atoms of nitrogen and two atoms of oxygen on each side of the equation.
Question 2:
How would you balance the reactant and products in the following decomposition reaction of hydrogen peroxide (H2O2) into water and oxygen gas?
H2O2 → H2O + O2
First make the inventory of atoms.
| Element | Reactants | Product |
| O | 2 | 3 |
| H | 2 | 2 |
Since the oxygen on the reactants side is in two different molecules this time, we do not have to change both. We can simply change the H2O on the products side. If we double the H2O, we get:
| Element | Reactants | Product |
| O | 2 | 4 |
| H | 2 | 4 |
H2O2 → 2H2O + O2
In order to balance the left side, we now simply need to double the H2O2
| Element | Reactants | Product |
| O | 4 | 4 |
| H | 4 | 4 |
2H2O2 → 2H2O + O2
Question 3
How would you balance the reactants and products in the following combustion reaction between propane (C3H8), which is used in BBQs, and oxygen (O2)?
First make the inventory of atoms.
| Element | Reactants | Product |
| C | 3 | 1 |
| H | 8 | 2 |
| O | 2 | 3 |
Let’s start by balancing the carbons. For this you would need to triple the number of carbons on the right side to get:
| Element | Reactants | Product |
| C | 3 | 3 |
| H | 8 | 2 |
| O | 2 | 7 |
C3H8(g) + O2(g) → 3CO2(g) + H2O
Next, let’s multiply the water on the right side by four to balance the hydrogen.
| Element | Reactants | Product |
| C | 3 | 3 |
| H | 8 | 8 |
| O | 2 | 10 |
C3H8(g) + O2(g) → 3CO2(g) + 4H2O
Finally, multiply the oxygen on the left side by five to balance the oxygen and you are done!
| Element | Reactants | Product |
| C | 3 | 3 |
| H | 8 | 8 |
| O | 10 | 10 |
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O
Learn More
Chemical Reactions Conditions and Speed
This backgrounder from Let's Talk Science discusses the special conditions that let some chemical reactions take place
Types of Chemical Reactions
This backgrounder from Let's Talk Science discusses the four main types of chemical reactions.
Evidence of Chemical Change
This backgrounder from Let's Talk Science discusses how to tell if a chemical reaction has taken place.
Balancing Chemical Equations (2015)
This video (4:17) by Professor Dave Explains gives a simple process by which chemical equations can be balanced.
References
Writing and Balancing Chemical Equations (n.d.) Chemistry. Retrieved from https://opentextbc.ca/chemistry/chapter/4-1-writing-and-balancing-chemical-equations/