Chemical Reactions: Conditions & Speeds
Electroplating equipment (Remigiusz Gora, iStockphoto)

Electroplating equipment (Remigiusz Gora, iStockphoto)
How does this align with my curriculum?
Learn about the special conditions that let some chemical reactions take place.
Chemical reactions happen all the time on Earth and in the Universe. But they only happen under certain conditions. That is a good thing! For example, fires only happen when there is enough oxygen and fuel. And can you imagine a world where matter didn’t decompose?
A chemical reaction involves the interaction of different substances or matter. These interactions involve energy. The energy can come in many forms, such as light, heat, or pressure. We will look at how these forms of energy work in chemical reactions in more detail.
Did you know?
Pressure is a form of kinetic energy.
Scientists describe chemical reactions using chemical equations. In a chemical equation, matter is identified in the reactants and products. The energy involved in the reaction is sometimes included in the equation. Let’s look at some examples of the different conditions needed for chemical reactions to happen.
Chemical Reactions Requiring Heat
Many reactions need a source of heat to happen. We call these reactions endothermic reactions. Most endothermic reactions can’t happen at normal room temperature. In the lab, scientists may heat reactants using bunsen burners. At home, you heat reactants in pots on the stove or in the oven.
Did you know?
Foods undergo chemical reactions when they cook.
The most obvious type of reaction that requires heat is a combustion reaction. In everyday language, we call this “burning.” Some combustion reactions are very obvious, such as when we burn wood. Other reactions are less obvious, such as when our bodies convert food into energy.
Did you know?
Not only do combustion reactions need heat, they also need oxygen.
Combustion is not the only type of chemical reaction that needs heat. Think about when you cook an egg. When you cook an egg, proteins in the egg break down because of the heat. We know this is a chemical reaction because the resulting products don’t have the same characteristics as the original. An uncooked egg white is liquid and transparent. A cooked egg white is solid and white. Cooking an egg is also an example of irreversible chemical reaction. You cannot uncook an egg!
Misconception Alert
When proteins are heated, bonds are broken through a process called denaturation. Not all denaturation processes are irreversible. It depends on how denatured the product is. This is the case for gelatine, like JELL-O™. Put liquid JELL-O™ in the fridge and it will become solid. Heat it up and it can return to its liquid form.
The breakdown of proteins in eggs when they are cooked is an example of a decomposition reaction. Decomposition reactions happen when a single reactant breaks down into two or more simpler products. E.g.,
AB → A + B
Another decomposition reaction requiring heat is the breakdown of calcium carbonate. You might not be familiar with the name of this compound, but it makes up coral reefs and the shells of shellfish.
CaCO3 → CaO + CO2
In this reaction, calcium carbonate breaks (CaCO3) down to form calcium oxide (CaO) and carbon dioxide (CO2). This reaction is happening more and more as oceans get warmer due to climate change. The warmer the ocean, the more fragile shells and corals become.
Try this!
In this hands-on activity you can explore what speeds up a chemical reaction using yeast.
Heat and Reaction Rate
Chemical reaction rate is the speed at which reactants become products. As a general rule, heat speeds up the rate of a reaction. Heat affects molecules by making them move faster. Imagine a room full of people. The faster they walk around, the more people they can meet in a minute. It’s the same for the molecules in a reaction. The faster they move, the easier it is for the reaction to take place. You also experience this when cooking. The higher you set the temperature, the faster the food will cook. It will not necessarily taste better though!
Reactions Requiring Light
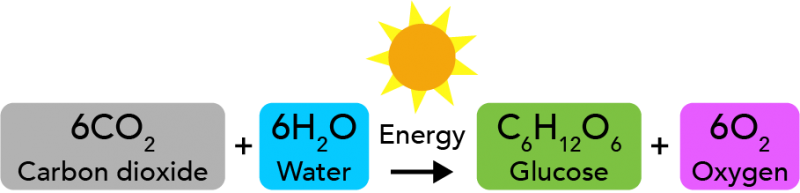
Light is another form of energy. Plants use energy from sunlight during the chemical reaction we call photosynthesis. In this chemical reaction, carbon dioxide and water react to form glucose and oxygen. Glucose is a type of sugar that plants use for their own energy.
Image - Text Version
During photosynthesis, six molecules of carbon dioxide react with six molecules of water to form one molecule of glucose (a sugar) and six molecules of oxygen. This reaction requires energy from the Sun or another source of light.
Some decomposition reactions also need light. An example of this that you might have around your house is hydrogen peroxide. People use hydrogen peroxide as a cleaner and disinfectant. When exposed to light, a chemical reaction takes place.
2H2O2 → 2H2O + O2
In this reaction, hydrogen peroxide (H2O2) decomposes to form water (H2O) and oxygen gas (O2). We don't want the reaction to break down in the bottle, which is why hydrogen peroxide often comes in an opaque brown bottle.
When someone tells you that you need to go outside to get some sunlight, do you ever wonder why? Sunlight helps our bodies form Vitamin D. It is a very important molecule in our bodies. It helps us to absorb calcium and phosphorus. These are both important minerals for teeth and bones. Our bodies do not produce Vitamin D on their own. They need a little help in the form of sunlight. In northern countries, like Canada, people may not get enough sunlight in the winter months. This is why eating food with added Vitamin D is a good idea at that time of year.
Reactions Requiring Pressure
Pressure has a similar effect on chemical reactions as temperature. The more that molecules compress, the closer together they are. The closer they are, the more chances they have of reacting with one another.
Gas molecules in our atmosphere also exert pressure down upon the Earth. Some of the chemical reactions on Earth are affected by this atmospheric pressure. One example of this is when carbon dioxide dissolves in oceans. Pressure makes this chemical reaction possible. The product of the reaction between carbon dioxide and water is carbonic acid. It makes the ocean more acidic. The increase in the acidity in oceans is known as ocean acidification. This process has always happened and usually reaches a natural chemical equilibrium. Living organisms have evolved to be able to tolerate those natural levels. However, global warming is speeding up this reaction. This is leading to challenges for animals in marine ecosystems.
Did you know?
Diamonds are formed when carbon is exposed to temperatures of more than 1 000 °C and pressures 50 000 times that of normal atmospheric pressure.
The effects of pressure on chemical reactions have not been studied as much as heat. There is a lot of exciting research happening about “high-pressure” chemistry.
Reactions Requiring Electricity
Electricity can also be used to break the bonds that hold together molecules. The most popular reaction requiring electricity is the electrolysis of water.
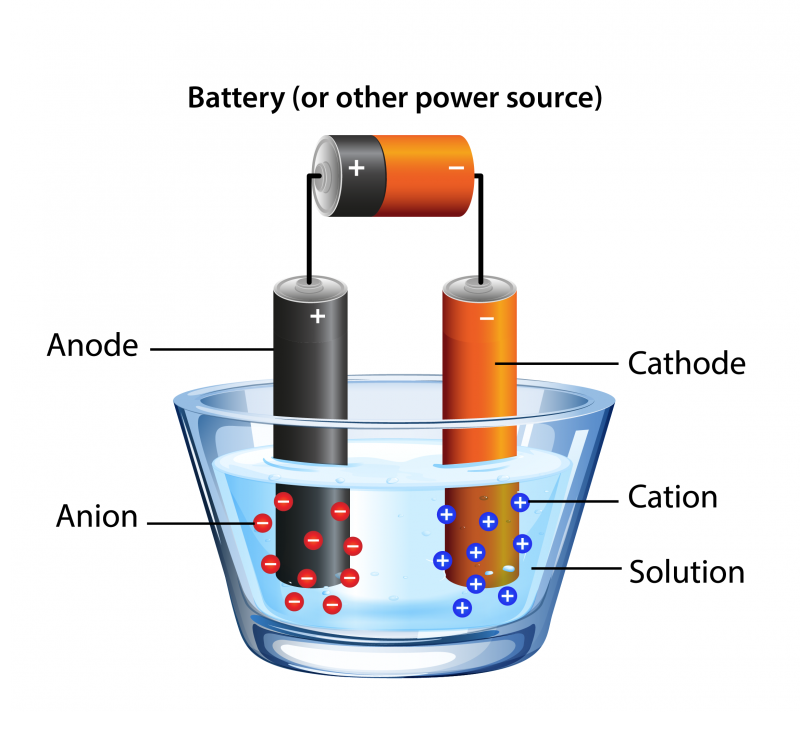
To do this, you need a source of electricity, like a battery. You need to connect the battery to two pieces of metal called electrodes. We call the positive electrode an anode and we call the negative electrode a cathode. The electrodes are placed in water, which completes the circuit. The charged electrode attracts charged particles, called ions, from the opposite charge. The anode attracts negative ions and the cathode attracts positive ions.
Image - Text Version
A glass dish is partly filled with water. Hanging in the solution are two pieces of metal. Both are connected via wires to a battery. When the circuit is closed, negatively-charged anions are attracted to the anode. Positively-charged cations are attracted to the cathode.
At the anode end, water molecules split to form oxygen gas (O2), hydrogen ions (H+) and electrons (e-). This type of reaction is called an oxidation reaction.
Note: The subscript “l” stands for liquid, the subscript “g” stands for gas and the subscript “aq” stands for an aqueous solution.
2 H2O(l) → O2(g) + 4 H+(aq) + 4e−
At the cathode end, hydrogen ions combine with electrons to form hydrogen (H2) gas.
2 H+(aq) + 2e− → H2(g)
This type of reaction is called a reduction reaction.
Did you know?
One way to remember the difference between oxidation reactions and reduction reactions is by remembering “LEO says GER.” This stands for Loss of Electrons is Oxidation, Gain of Electrons is Reduction.
Another common reaction involving electricity is electroplating. In this process, a thin layer of one metal is applied over another layer of metal. Electroplating is often used to apply a thin layer of a valuable metal, such as gold or silver, onto objects like jewelry and cutlery. Let’s see how it works.
If we wanted to electroplate something, we would need:
- Two electrodes made from materials that conduct electricity, such as metal;
- An electrolyte, which is a solution that conducts electricity; and
- A source of electricity.
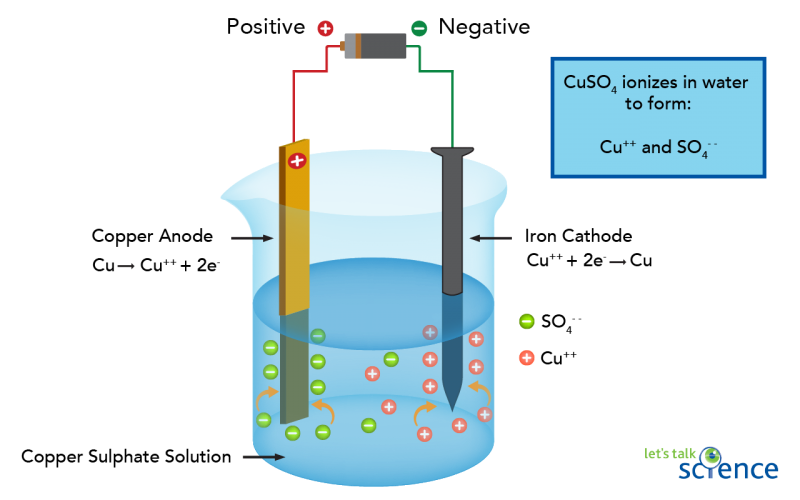
Say, we wanted to plate an iron nail with copper. We would need a copper electrode, an iron electrode and an electrolyte, such as a solution of copper sulphate (CuSO4 (aq)). If we put the two electrodes into the solution and connected up the circuit to a battery, the copper would become the anode and the iron nail would become the cathode. When electricity flows through the circuit, the copper sulphate would split into copper ions (Cu++) and sulphate ions (SO4--). The copper ions would be attracted to the negatively charged iron nail and form a thin layer of copper on the nail. Meanwhile, the negatively-charged sulphate ions would be attracted to the positively-charged copper anode. These extra electrons then move through the wires to the battery, and back down to the iron nail.
Image - Text Version
A glass beaker is partly filled with a pale, blue solution of copper sulphate. Hanging in the solution is a rod of copper and an iron nail. Both are connected via wires to a battery. When the circuit is closed, Ions of sulphate are attracted to the copper anode. Ions of copper are attracted to the iron cathode.
This is not a quick process, but as long as there are ions, and electrons keep moving, the process can take place.
Catalysts
Some chemical reactions involve catalysts. Catalysts are substances that help a reaction without being used up in the reaction itself. They work by reducing the amount of energy required for the reaction to take place. Remember our peroxide decomposition reaction? You can speed up this reaction by adding yeasts that have a special catalyst.
Catalysts are important in many industrial processes. They make reactions happen faster, which often makes them cheaper. And, since they are not used up, they can be used over and over. This principle is behind Catalytic converters, which help keep toxic air pollution from coming out of vehicle tailpipes.
A special type of catalyst that is essential to our lives are enzymes. Enzymes speed up all kinds of chemical reactions in our bodies. This includes things like how we use oxygen to how we grow. Unlike most industrial catalysts, which are simple compounds, enzymes are complex proteins.
Sometimes it’s complicated!
Ammonia (NH3) is one of the most commonly produced chemicals by humans. It is essential in producing fertilizers and pharmaceutical products. It is also present in many cleaning products. It is even being thought about as a possible green fuel. Ammonia is produced by combining atmospheric nitrogen (N2) and hydrogen (H2) gases.
N2(g) + 3H2(g) → 2NH3(g)
To make this happen, you need high temperature, high pressure and a catalyst!
Learn More
How to speed up chemical reactions (and get a date) (2012)
This video (4:56 min.) by TedEd explains the different ways to speed up chemical reactions
Electroplating (2015)
This video by DeWitt (15:16 min.) explains how electroplating works.
Electrolysis of Copper Sulphate Using Copper Electrodes
This video by Electrical4U (1:48 min.) has a simple explanation of the electrolysis of copper sulphate using copper electrodes.
7 things you may not know about catalysis
This article by the Argonne National Laboratory explains why catalysts are important compounds.
Examples of Catalysts
This page by Your Dictionary gives examples of important catalysts.
Conditions Required in Chemical Reaction
This page by Kul Labs presents the conditions for chemical reactions, along with the equation examples.
References
Dillon, S.R. (n.d.) The Rates of Chemical Reactions. Chemistry For Liberal Studies - Forensic Academy. Retrieved from https://www.chem.fsu.edu/chemlab/chm1020c/Lecture%208/01.php
Electrolysis: Using Electricity to Do Chemistry. (2019, July 1). Retrieved from https://chem.libretexts.org/@go/page/161969