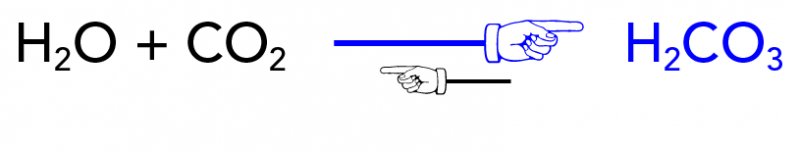
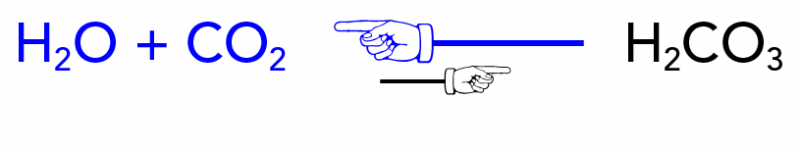
The Chemistry of Pop

A person opens a can of pop (explosivekeeper, iStockphoto)

A person opens a can of pop (explosivekeeper, iStockphoto)
How does this align with my curriculum?
Curriculum Alignment
AB
11
Knowledge and Employability Science 20-4 (2006)
Unit A: Applications of Matter and Chemical Change
BC
11
Chemistry 11 (June 2018)
Big Idea: Solubility within a solution is determined by the nature of the solute and the solvent.
NU
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit A: Applications of Matter and Chemical Change
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Solubility within a solution is determined by the nature of the solute and the solvent.
YT
12
Chemistry 12 (British Columbia, June 2018)
Big Idea: Saturated solutions are systems in equilibrium.
NT
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit A: Applications of Matter and Chemical Change
NT
10
Knowledge and Employability Science 10-4 (Alberta, 2006)
Unit A: Investigating Properties of Matter
BC
12
Chemistry 12 (June 2018)
Big Idea: Dynamic equilibrium can be shifted by changes to the surrounding conditions.
YT
12
Chemistry 12 (British Columbia, June 2018)
Big Idea: Dynamic equilibrium can be shifted by changes to the surrounding conditions.
BC
11
Chemistry 11 (June 2018)
Big Idea: The mole is a quantity used to make atoms and molecules measurable.
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: The mole is a quantity used to make atoms and molecules measurable.
BC
10
Science Grade 10 (March 2018)
Big Idea: Energy change is required as atoms rearrange in chemical processes.
BC
12
Chemistry 12 (June 2018)
Big Idea: Acid or base strength depends on the degree of ion dissociation.
NU
12
Chemistry 30 (Alberta, 2007, Updated 2014)
Unit D: Chemical Equilibrium Focusing on Acid-Base Systems
NU
10
Science Grade 10 (British Columbia, June 2016)
Big Idea: Energy change is required as atoms rearrange in chemical processes.
ON
10
Science Grade 10 Applied (SNC2P)
Strand C: Chemical Reactions and Their Practical Applications
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Matter and energy are conserved in chemical reactions.