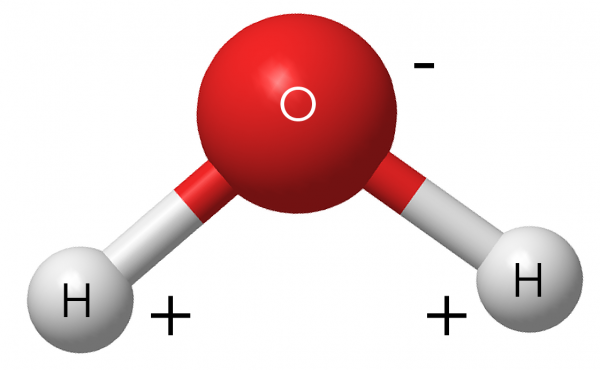
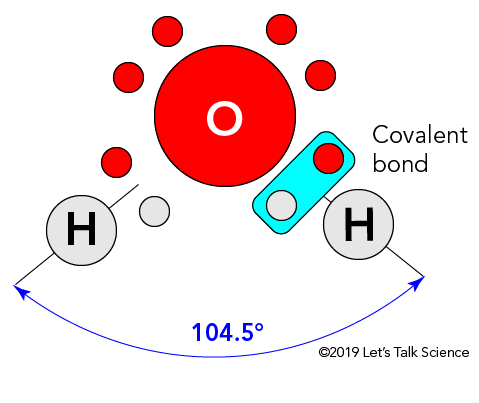
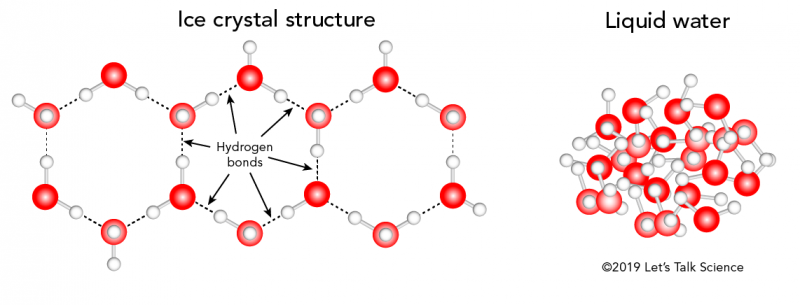
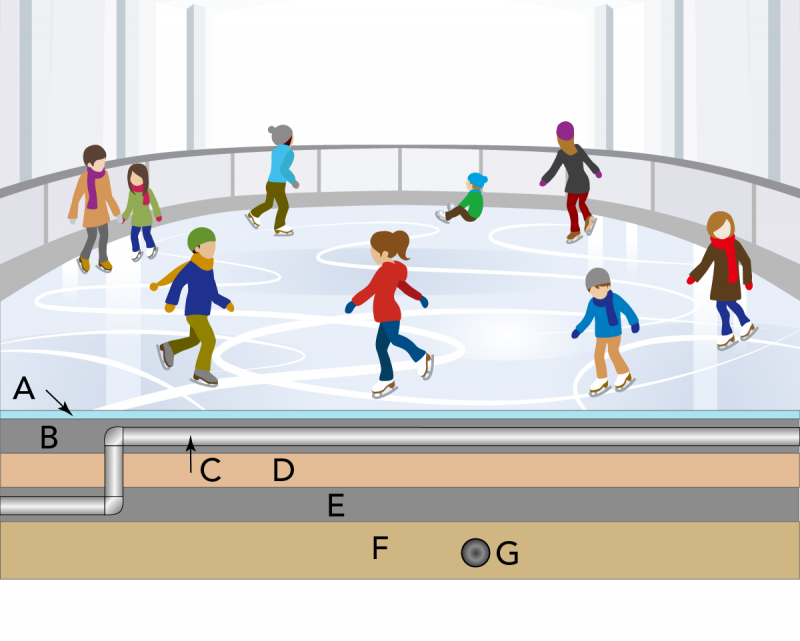
Why Do Ice Rinks Stay Frozen?

People playing ice hockey (VisualCommunications, iStockphoto)

People playing ice hockey (VisualCommunications, iStockphoto)
7.61
How does this align with my curriculum?
Curriculum Alignment
BC
8
Science Grade 8 (June 2016)
Big Idea: The behaviour of matter can be explained by the kinetic molecular theory and atomic theory.
YT
8
Science Grade 8 (British Columbia, June 2016)
Big Idea: The behaviour of matter can be explained by the kinetic molecular theory and atomic theory.
NU
2
K-6 Science and Technology Curriculum (NWT, 2004)
Matter and Materials: Properties of Liquids and Solids
NU
2
K-6 Science and Technology Curriculum (NWT, 2004)
Earth and Space Systems: Air and Water in the Environment
ON
2
Science and Technology, Grades 2 (2022)
Strand C. Matter and Energy; Properties of Liquids and Solids
ON
2
Science and Technology, Grades 2 (2022)
Strand E. Earth and Space Systems; Air and Water in the Environment
NT
2
K-6 Science and Technology Curriculum (NWT, 2004)
Matter and Materials: Properties of Liquids and Solids
NT
2
K-6 Science and Technology Curriculum (NWT, 2004)
Earth and Space Systems: Air and Water in the Environment
AB
3
Science 3 (2023)
Matter: Understandings of the physical world are deepened by investigating matter and energy.
ON
11
Chemistry, Grade 11, University (SCH3U)
Strand B: Matter, Chemical trends, and Chemical Bonding
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Atoms and molecules are building blocks of matter.
BC
11
Chemistry 11 (June 2018)
Big Idea: The mole is a quantity used to make atoms and molecules measurable.