Hydrogen Fuel Cell Vehicles

Back of a car with a hydrogen fuel cell (gchutka, iStockphoto)

Back of a car with a hydrogen fuel cell (gchutka, iStockphoto)
How does this align with my curriculum?
Learn about vehicles that use hydrogen for fuel.
The Scoop on Hydrogen
Hydrogen is the most common chemical element in the Universe. It makes up about three quarters of all matter. But did you know, like gasoline and diesel, hydrogen can be used as a fuel in vehicles? Let’s find out how.
Where does hydrogen come from?

Diagram showing different molecules of hydrogen, oxygen and carbon (Source: Let’s Talk Science using an image by :LoopAll via iStockphoto).
Image - Text Version
Shown is a series of colour illustrations of molecules.
There are three rows with three small, labelled, illustrations each.
Starting on the top left, the first image is labelled "Oxygen." It has one red sphere marked with the letter O. The second image labelled "Hydrogen." It has a single blue sphere marked with the letter H. The third is labelled "Carbon." It has a single black sphere marked with the letter C.
In the second row, the first illustration is labelled "Oxygen gas, O2." This has two red spheres joined together with a white stick. The second image is labelled "Hydrogen gas, H2." It has two blue spheres joined together with a white stick. The third image is labelled "Water, H2O." It has two blue spheres, joined to a red one between them, with a white stick each.
In the third row, the first image is labelled "Carbon dioxide, CO2." It has two red spheres joined to a black one between them. These have two white sticks at each of the two joins. The second image is labelled "Ethane, C2, H6." It has two black spheres joined with one white stick. Three blue spheres are joined to one black sphere, with single sticks. Two blue spheres are joined to the second black one with single sticks. The final image, on the bottom right, is labelled Methane, CH4. Here, four blue spheres are joined to one black one, with single sticks.
In order to use hydrogen as a fuel for vehicles, it must be in its pure form. This means that we must break apart other molecules to get the hydrogen out.
We can get hydrogen using different types of chemical reactions.

Steam coming out of smokestacks (Source: acilo via iStockphoto).
Image - Text Version
Shown is a colour photograph of white steam billowing from two tall towers above a large building.
The towers are tall, cylindrical, and grey on the bottoms and white on the tops. The clouds of steam are blowing to the right, across the greyish-blue sky behind.
The building at the base of the towers is wide and grey. It has a long row of windows, and large air ducts. In the foreground are dense, leafy trees, starting to turn yellow.

Hydrogen production plant that gets its energy from Wind turbines (Source: Aranga87 via iStockphoto).
Image - Text Version
Shown is a colour photograph of buildings, wind turbines, and three tanks marked H2.
In the foreground are three, long cylindrical white tanks labelled H2. They are in a row next to two pale green buildings with dark roofs. Just behind, there are three large silver structures with domed roofs. In the background, three tall, thin wind turbines stretch above the horizon. The sky behind is bright blue with a few grey and white clouds.
Hydrogen-powered Vehicles
There are two kinds of hydrogen-powered vehicles:
- Hydrogen fuel cell vehicles (FCEVs)
- Hydrogen internal combustion engines (Hydrogen ICE)
How do hydrogen fuel cell vehicles work?
A hydrogen fuel cell vehicle uses a fuel cell to generate electricity. It uses the electricity to run an electric motor much like the motor in an electric vehicle.
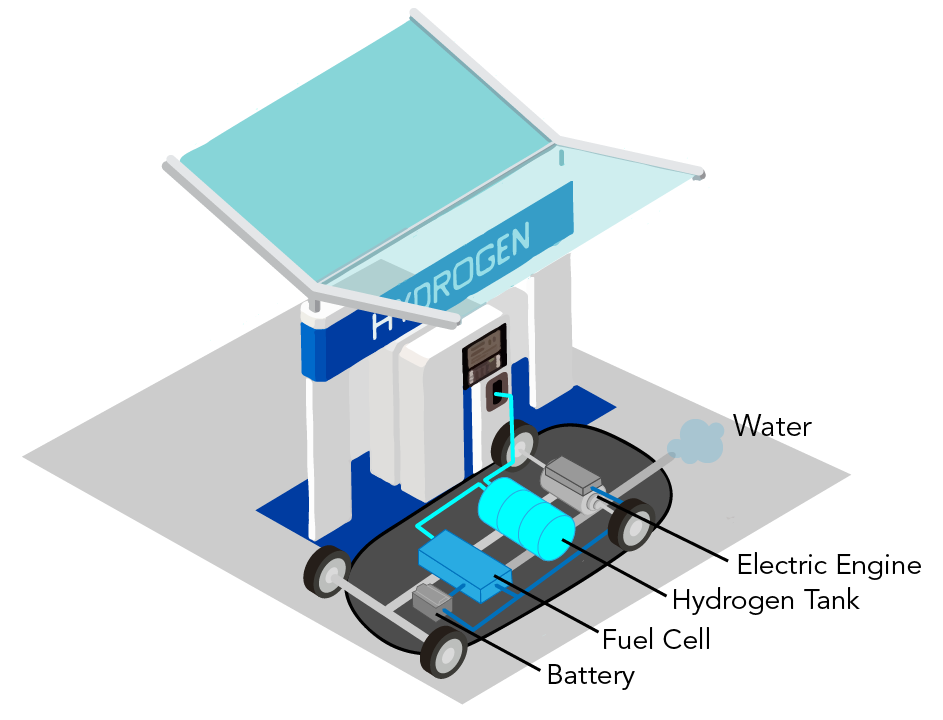
Hydrogen fuel cell car at a hydrogen filling station (Let’s Talk Science using an image by Aranga87 via iStockphoto).
Image - Text Version
Shown is a colour illustration of the parts inside a car at a filling station with a "Hydrogen" sign.
The station looks like a typical gasoline filling station. It has a large white structure in the centre, with space for cars to pull up on either side. A sign at the top reads "Hydrogen."
A car is pulled up on the nearest side. Its top parts are removed to show the parts inside. Starting at the front, there is a grey box labelled "Battery" on the right. Further back, in the centre, is a large blue rectangle labelled "Fuel Cell." In the middle of the car is an even larger cylindrical barrel, labelled "Hydrogen Tank." Between the rear wheels is a long grey shape labelled "Electric Engine." A tailpipe spews a pale blue cloud out the back of the car. This is labelled "Water."
Where the gas pump would be, a teal line runs from the station, to one side of the hydrogen tank. Another teal line runs from here to the fuel cell. A blue line runs from the fuel cell to the battery. Another blue line runs from the battery to the engine.
How do hydrogen internal combustion engines work?
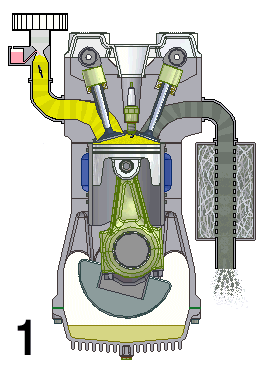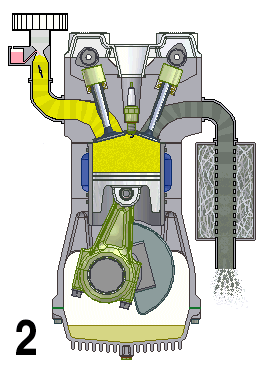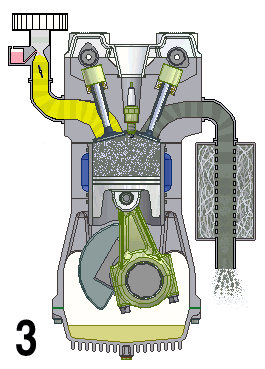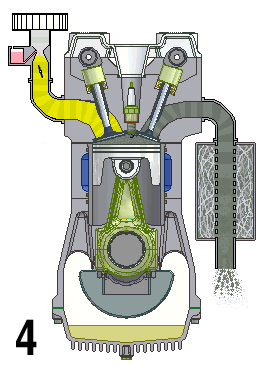
This animation shows the four steps that occur in a combustion engine. The fuel enters at the left and the waste gases exit at the right (Source: UtzOnBike (derivative work) via Wikimedia Commons).
Animation - Text Version
Shown is a colour animated GIF that shows what happens inside an internal combustion engine.
In the bottom left corner, black numbers cycle through 1, 2, 3, and 4 on a loop.
On the top left, a yellow substance flows through a pipe. At the bottom of the pipe, a grey stopper on the end of a moving pole, moves up and down, blocking, then allowing the liquid to move into a chamber below.
When the chamber is full of yellow liquid, a white spark from a green plug in the roof of the chamber turns the chamber red. The chamber then quickly turns grey.
When the chamber is grey, a stopper on the top right of the chamber lets the grey substance up through a pipe. The end of this pipe spews grey fumes, like the exhaust pipe of a car.
At the bottom of the chamber, wide grey object moves up and down in a steady rhythm. It moves down when the chamber becomes yellow, then up until the spark, then down when it's grey.
A large, round green object with a pointed triangular top, hangs from a hinge on the bottom side of the wide grey object. A grey half-circle attached to it moves smoothly around an empty chamber.
There is a downside to this type of engine. The heat from the reaction causes nitrogen from the air to combine with oxygen. The reaction looks like this:
H2 + O2 + N2 → H2O + NOx
The products of the reaction are water and nitrogen oxides (NOx). These chemicals are harmful to both people and the environment. This makes hydrogen internal combustion engines less environmentally-friendly than hydrogen fuel cells.
Did you know?
Toyota successfully completed a 24 hour endurance race with a hydrogen combustion engine car.
Which type of hydrogen-power is better?

Hydrogen tank on the side of a heavy transport truck (Source: Scharfsinn86 via iStockphoto).
Image - Text Version
Shown is a colour photograph of a silver tank marked H2, strapped to the back of a large truck.
The camera is looking at the back of a transport truck, without a shipping container on it. The tank is fastened horizontally, below where the container would sit. It fits the space between the front and middle sets of wheels.
Misconception alert!
Some people think the hydrogen in fuel cell cars is dangerous. This is because hydrogen gas is flammable . In fact, hydrogen fuel cell vehicles are not more dangerous than regular vehicles. It is even possible that they are safer! If a hydrogen tank is damaged, the hydrogen quickly moves off into the air. This makes starting a fire unlikely. If a gasoline tank is damaged, the gasoline fumes do not move off. This is why the risk of fires at vehicle accidents is very high.
Hydrogen Fuel Cell Vehicles: Challenges and Opportunities
Hydrogen fuel cell vehicles are a great zero-carbon option. So, why are gas-fueled cars still the most common vehicles on the road? Let’s look at some of the challenges with getting hydrogen fuel cell vehicles on the road in Canada.
The Challenges
For one thing, there will need to be new infrastructure. This includes building places to make hydrogen. It also includes building hydrogen storage facilities and hydrogen refuelling stations.
Hydrogen fuel cell vehicles are also more expensive to make than regular gasoline-fueled vehicles. This means that they will be expensive to buy at first. As more people buy them, the cost for them should go down.
Hydrogen fuel cells and hydrogen combustion engines are still fairly new technologies. In order to take them into the mainstream, more support needs to go to research and testing. This can come from both governments and private companies.
Currently there is not a lot of hydrogen that has been sourced in a carbon-neutral way.
More work needs to be done on creating supplies of hydrogen. Especially those that are made without using fossil fuels.
Right now not many people know about the benefits of hydrogen-powered vehicles. This includes both the general public as well as those in industries and government.
Once people know more about this technology, they may buy vehicles and encourage others to fund research.
Despite the challenges, hydrogen-powered vehicles provide many opportunities.
The Opportunities
Canada has everything it needs to make hydrogen-powered vehicles. It has many skilled people and places for them to work. Canada also generates a lot of its electricity using renewable sources of energy.
To become a leader in this area, Canada will need support from industries, governments and the public.
Canada already has ways of distributing and storing hydrogen. This includes using existing oil and gas pipelines. However, changing pipelines over to hydrogen will take time and money.
Like with any fuel, there will need to be a network of refuelling stations. Creating this network will provide many jobs for Canadians.
Canada is a large country. The people who live there must drive many kilometres to get from place to place. Hydrogen can play an important role in getting the vehicles people need off of fossil fuels. Changing to hydrogen can be a solution to reducing greenhouse gas emissions. This will help to fight climate change.
Did you know?
10.5% of Canada’s greenhouse gas emissions come from freight transportation.
What’s Next?
People will not stop needing to drive, but the fuel they use could make a big difference to the environment. Using hydrogen could lead to fewer carbon emissions. There is even the potential to create a carbon-neutral future. Imagine a future where the only emissions created by vehicles are 100% water!
Follow the link to learn more about how Canada plans to reach net-zero emissions by 2050.
Learn More
I try LIVING with a HYDROGEN FUEL CELL car… (2021)
This video (8:46 mins) from Downie Live shows what it’s like to live with a hydrogen fuel cell car for a day!
Having a Gas with Water
This hands-on activity from Exploratorium is a great way for students to use electricity to break water into its elemental components.
Indicating Electrolysis
Another hands-on activity from Exploratorium that allows students to break up water into hydrogen and oxygen gas with a simple electrolysis device, and use an acid-base indicator and a magnet to create groovy swirls of colour.
Toyota's Developing A Hydrogen Combustion Engine! (2021)
This Engineering Explained video (14:05 mins) provides an in-depth explanation of Toyota’s nearly carbon-neutral hydrogen combustion engine.
How do fuel cell electric vehicles work? (2020)
This video (1:43 mins) from Volvo Group Videos explains how fuel cell vehicles work.
References
Environment and Natural Resources Canada (2022). Net-Zero Emissions by 2050.
Let's Talk Science (2022). Earth Month: Green Energy.
Natural Resources Canada (2022). Energy from hydrogen: the basics.
Natural Resources Canada (2020). Hydrogen opportunities: Key findings.
Natural Resources Canada (2022). Producing hydrogen in Canada.
Natural Resources Canada (2021). Remaining Challenges.
Natural Resource Canada (2022). Using hydrogen in Canada.
Nebergall, J. (Jan 26, 2022). How do hydrogen engines work? Cummins.
Nebergall, J. (Jan 27, 2022). Hydrogen internal combustion engines and hydrogen fuel cells. Cummins.
U.S. Department of Energy (n.d.) Hydrogen Production Processes. Office of Energy Efficiency & Renewable Energy.