Types of Chemical Reactions

Heating a liquid over a flame in a lab (AtnoYdur, iStockphoto)

Heating a liquid over a flame in a lab (AtnoYdur, iStockphoto)
How does this align with my curriculum?
Learn about the four main types of chemical reactions.
Chemical reactions take place around us, and even inside us, every day. There are so many different chemical directions that scientists find it is helpful to classify them into groups. There are four main types of chemical reactions:
- Synthesis
- Decomposition
- Replacement (also known as Displacement)
- Combustion.
But before reading this, we strongly suggest you get familiar with the way we write chemical equations by reading the backgrounder on chemical equations. This will allow you to better understand the examples used below to explain the different types of reactions.
Synthesis Reactions
In a synthesis reaction, two or more atoms or molecules combine to form a compound. In other words, two or more reactants come together to form a product. We can use the symbol A to represent one of the reactants and B to represent the other reactant. In a synthesis reaction, A and B combine to form AB.
The general formula for a synthesis reaction is:
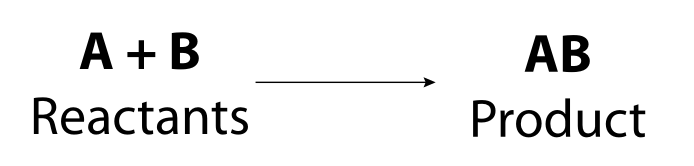
Image - Text Version
Shown is a general chemical formula. In it, reactant A combines with reactant B to form product AB.
The physical and chemical properties of A, B and AB are different. An example of a synthesis reaction is the formation of water (H2O). This reaction occurs when hydrogen gas (H2) reacts with oxygen (O2) gas. The resulting product is liquid water.
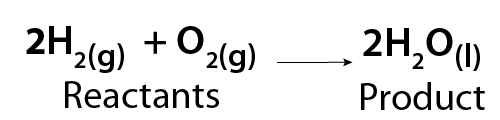
Image - Text Version
Shown is a chemical equation. It in, two molecules of hydrogen gas (2H2) combine with one molecule of oxygen gas (02) to form two molecules of liquid water (2H2O).
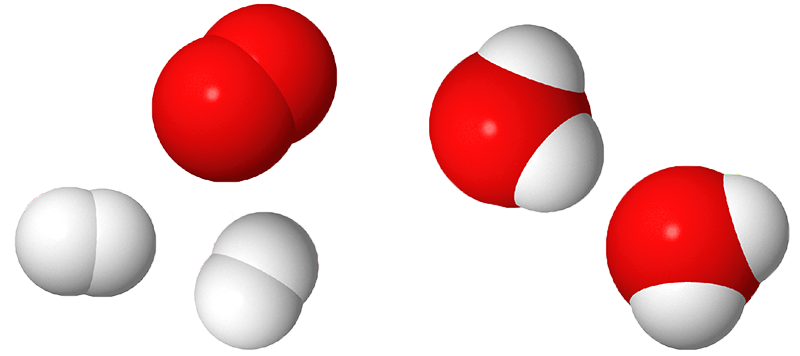
Image - Text Version
Shown are 3D renderings of hydrogen gas, oxygen gas and water molecules. Hydrogen is represented by white, somewhat spherical shapes and oxygen is represented by red, somewhat spherical shapes.
Molecules of hydrogen gas have two of the white shapes stuck together. Two set of these are shown. Molecules of oxygen gas have two of the red shapes stuck together. One set of these is shown. Molecules of water have two of the white shapes stuck to one of the red shapes. Two sets of these are shown.
This is a pretty incredible reaction when you think about it. Hydrogen and oxygen gases are both highly combustible gases. But when combined, their product is a substance that can put out fires!
Not all synthesis reactions result in the combination of two substances into one. Some synthesis reactions can result in more than one product.
Photosynthesis is one of the most important chemical reactions on Earth. It allows plants and some microbes to convert water and carbon dioxide gas into storable sugar and oxygen. This oxygen is critical for life on Earth!
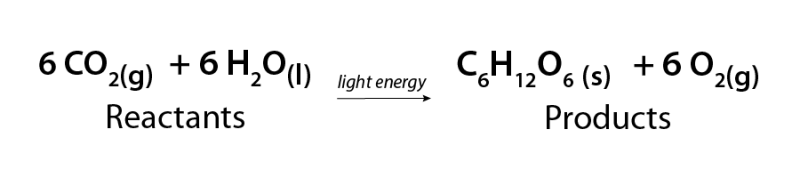
Image - Text Version
Shown is a chemical equation. In it, six molecules of carbon dioxide gas (6CO2) combine with six molecules of water (6H2O) in the presence of light energy to form one molecule of glucose (C6H12O6) and six molecules of oxygen gas (6O2).
Here is a cool synthesis reaction that is sometimes called the Pharaoh's snake. In this reaction, calcium gluconate (C12H22CaO14) reacts with oxygen (O2) in the presence of a heat source to form water (H2O), carbon dioxide (CO2), calcium oxide (CaO) and carbon (C).
Decomposition Reactions
Decomposition reactions are essentially the reverse of synthesis reactions. This type of reaction involves a compound that breaks apart into two or more atoms or molecules. This often occurs when atoms or molecules are heated. AB is broken down to form A and B. Similar to synthesis, the physical and chemical properties of AB, A, and B are different.
The general equation for a decomposition reaction is:
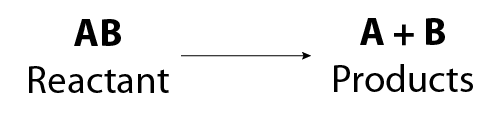
Image - Text Version
Shown is a general chemical equation. In it, reactant AB breaks apart to form products A and B.
A specific example of this reaction is the breakdown of water into hydrogen and oxygen gas. This happens when electricity passes through the water in a process called electrolysis.
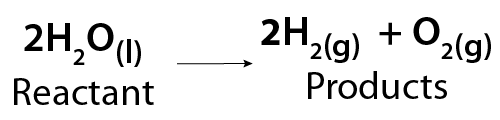
Image - Text Version
Shown is a chemical equation. In it, two molecules of liquid water (2H2O) break down to form two molecules of hydrogen gas (2H2) and one molecule of oxygen gas (O2).
An important decomposition reaction happens when we bake some of our favourite foods, like cookies. Many recipes include baking soda as an ingredient. Baking soda, or sodium bicarbonate NaHCO3(s), is used to make foods moist and fluffy. But, how does it work?
When the cookie batter is put in the oven, heat is added to the mixture. When the temperature of the batter reaches temperatures above 80℃ (176°F), the baking soda breaks down into sodium carbonate (Na2CO3), water vapour (H20), and carbon dioxide gas (CO2).

Image - Text Version
Shown is a chemical reaction. In it, two molecules of sodium bicarbonate (2NaHCO3) break down in the presence of heat to form one molecule of solid sodium carbonate (Na2CO3), one molecule of carbon dioxide gas and one molecule of water vapour.
Did you know?
Sodium carbonate is also known as soda ash. Read more about soda ash in this backgrounder.

Image - Text Version
Shown is a colour photograph of a tray of cookies on a cookie sheet in an oven. The cookies are golden brown and ready for eating!
The bubbles of gas get trapped by the flour mixture as it hardens, giving us the fluffy cookies we love!
Replacement Reactions
As the name suggests, in a replacement reaction one element replaces another element. There are two types of replacement reactions. These are single replacement reactions and double replacement reactions.
Single Replacement Reactions
In a single replacement reaction, a single uncombined element C replaces another element in a compound AB. In this case, two reactants yield two products.
The general equation for a single replacement reaction is:
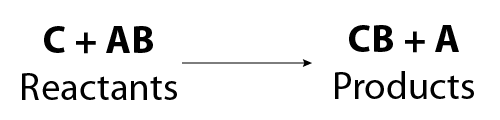
Image - Text Version
Shown is a general chemical equation. In it, reactant C combines with reactant AB to form product CB and product A.
An example of a simple replacement reaction is the explosive reaction that happens when potassium reacts with water.
In this reaction, potassium (K) reacts with water (H2O). This reaction produces hydrogen gas and solid potassium hydroxide (KOH) in solution.
The equation for the reaction between potassium and water is:
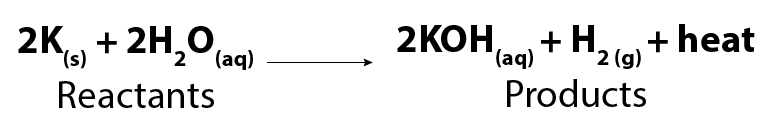
Image - Text Version
Shown is a chemical formula. In it, two molecules of solid potassium (2K) react with two molecules of liquid water (2H2O) to form two molecules of potassium hydroxide (2KOH) in solution and one molecule of hydrogen gas (H2). The reaction also releases energy as heat.
The reaction between potassium and water also releases energy in the form of heat. This makes it an exothermic reaction. Another reaction happens soon after the hydrogen gas is produced. Heat from the reaction of potassium and water causes the hydrogen gas to combust (see more about combustion reactions below).
Double Replacement Reactions
Now that you know what happens in a single replacement reaction, what do you think happens in a double replacement reaction? If you thought that two parts switched then you would be right!
In a double replacement reaction, two reactant compounds form two new product compounds through the exchange of ions. Ions are charged molecules or atoms. Cations are positive ions (+) and anions are negative ions (-). Only cations can replace cations and only anions can replace anions.
The general formula for a double replacement reaction is:
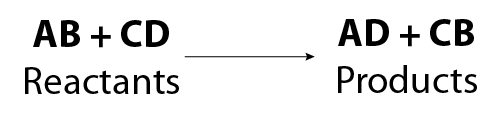
Image - Text Version
Shown is a general chemical formula. In it, reactant B replaces reactant D and reactant A replaces reactant C.
In this case A and C are cations and B and D are anions. Therefore only A can replace C and only B can replace D.
The most well-known and widely used double replacement reactions are acid-base reactions.
Acid-Base Reactions
An acid–base reaction is a special type of double replacement reaction that occurs between an acid and a base. Acids are substances that are close to 0 on the pH scale. Acidic foods have a sour taste. This includes things such as vinegar and lemon juice. Bases are substances that are close to 14 on the pH scale. Bases are most often seen in our cleaning cupboards. This includes things such as bleach and detergent.
Did you know?
The pH scale gives a measurement of the amount of H+ in a solution. You can explore acids and bases in this hands-on activity.
Typically, an acid-base reaction results in the formation of a precipitate.
Acids and bases have a very unique and interesting relationship. When they react together, each is able to cancel out, or neutralize, the acidic or basic properties of the other. That is why acids and base reactions are also called neutralization reactions.
In many neutralization reactions, a positive hydrogen ion (H+) in an acid reacts with a negative hydroxide ion (OH-) ion in a base, forming water.
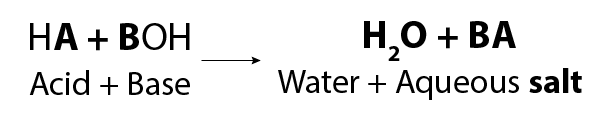
Image - Text Version
Shown is a general chemical formula. In it, an acid HA reacts with a base BOH to form water (H2O) and an aqueous salt BA.
It is important to note that the word “salt” is a general term which applies to the products of all such acid-base reactions. The table salt that we eat can be produced through this reaction. You can make sodium chloride, better known as table salt, by reacting hydrochloric acid and sodium hydroxide.
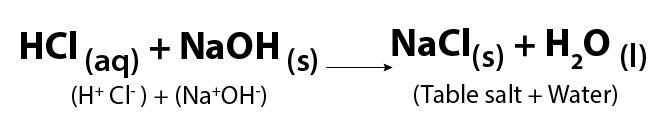
Image - Text Version
Shown is a chemical formula. In it, aqueous hydrochloric acid (HCl) and solid sodium hydroxide (NaOH) react to form solid sodium chloride (NaCl) and liquid water (H2O). Sodium chloride is also identified as table salt.
See this reaction in action by watching the video below:
Combustion Reactions
A combustion reaction occurs when oxygen gas (O2) reacts with certain types of compounds. We often call the other compound fuel. A more familiar term for combustion reactions is burning. The most common products of combustion reactions are carbon dioxide and water. Water is rarely seen as it is its gaseous form.
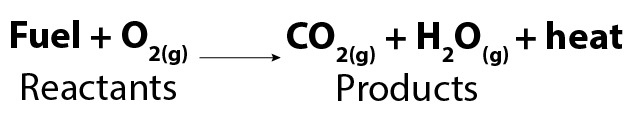
Image - Text Version
Shown is a chemical formula. In it, fuel combines with oxygen gas (O2) to form carbon dioxide gas (CO2), water vapour (H2O) and heat. The fuel and oxygen gas are identified as reactants. The carbon dioxide gas and water vapour are identified as products.
When a fuel reacts in such a way that only carbon dioxide and water are produced, we call it a complete combustion reaction.
The burning of butane, which is used in lighters, is an example of complete combustion. The chemical equation for this is:
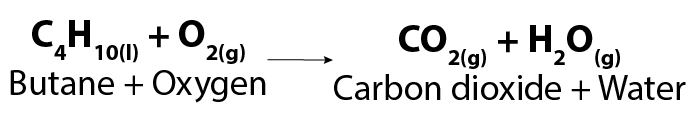
Image - Text Version
Shown is a chemical formula. In it, one molecule of butane (C4H10) reacts with oxygen gas (O2) to form carbon dioxide gas (CO2) and water vapour (H2O).
Sometimes fuel does not completely react. In this case we call it an incomplete combustion reaction. Other products that result from the incomplete combustion of carbon-based fuels. These include carbon monoxide, a dangerous odorless gas and soot, which are particles of carbon.
Let’s see how combustion works when a candle burns.
When you apply a flame to the wick of a candle, a combustion reaction starts to happen. Most candle wicks are made from cotton, which burns easily. The burning wick gets its oxygen from the air. Without oxygen, the wick will stop burning. The heat produced during this combustion reaction will melt the solid wax and turn it into a gaseous form of the wax. The tiny gaseous molecules of wax then undergo their own combustion reaction with the oxygen in the air. The major products are carbon dioxide and water vapour. There is also considerable soot, made from carbon atoms, as well as some carbon monoxide (CO).
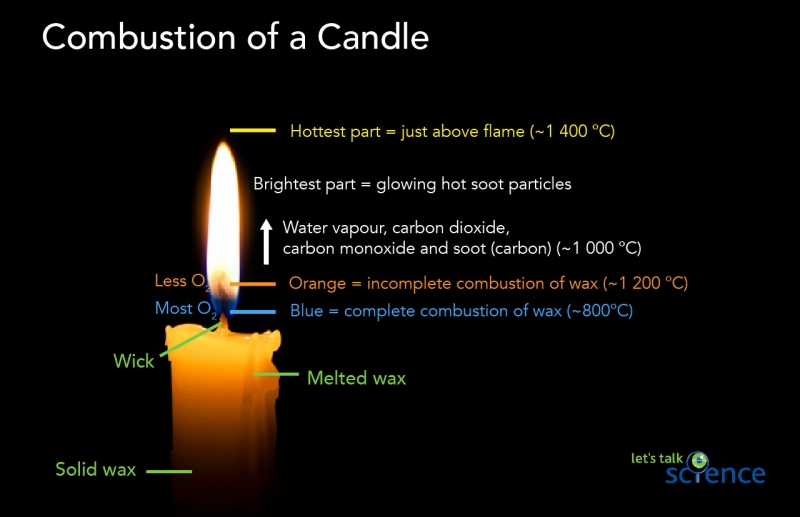
Image - Text Version
Shown is a photograph of a candle burning against a black background. Overlaid on the image are labels identifying different parts of the candle and candle temperatures.
The bottom of the candle is labelled as being made of solid wax. The upper part of the candle, closest to the flame, is labelled as being melted wax. At the top of the candle is a narrow, vertical object identified as the wick. The wick is the colour of the wax at the bottom and black at the top.
The colours of the flame are labelled. The lowermost part of the flame is blue. Blue signifies the complete combustion of wax. This occurs at approximately 800 degrees Celsius. This part of the flame is able to react with most oxygen gas.
Above the blue part of the flame is the orange part of the flame. Orange signifies the incomplete combustion of wax. This occurs at approximately 1200 degrees Celsius. This part of the flame reacts with less oxygen gas than the blue part of the flame.
Above the orange part of the flame is the pale yellowish-white part of the flame. This is identified as the brightest part of the flame. The light is caused by glowing hot soot particles. This region of the flame is made of water vapour, carbon dioxide, carbon monoxide and soot (carbon). The temperature of the flame in this region is around 1000 degrees Celsius.
Just above the top part of the flame, is the hottest part of the flame. We cannot actually see this part. It has a temperature of around 1400 degrees Celsius.
A candle flame is a good example of incomplete combustion. The engines in vehicles also undergo incomplete combustion when they burn gasoline and diesel fuel.
Did you know?
In microgravity, a candle shape is round because there is no upward movement of hot gases.
What is important to know about combustion reactions is that they are exothermic reactions.This means they produce heat. People often use combustion reactions for the heat and light that these reactions produce. Whether it is burning wood to create a toasty campfire, using natural gas to heat food and homes, or the combustion of acetylene with oxygen in a welder’s torch, combustion reactions play an important role in our lives.
Actually…
ALL types of chemical reactions play an important role in our lives!
Learn More
Acids and Bases Are Everywhere
This page and following one from Chem4Kids present the acid and base reactions.
Intro to Chemistry: Types of Chemical Reactions (part 1)
This video from chemistry teachers Jonathan Bergmann and Aaron Sams presents live demonstrations of synthesis reactions.
Intro to Chemistry: Types of Chemical Reactions (part 2)
This video from chemistry teachers Jonathan Bergmann and Aaron Sams presents live demonstrations of decomposition and single replacement reactions.
Intro to Chemistry: Types of Chemical Reactions (part 3)
This video from chemistry teachers Jonathan Bergmann and Aaron Sams presents live demonstrations of double replacement and combustion reactions.
References
Candle Science (n.d.) Candles.org. Retrieved from https://candles.org/candle-science/
Combustion and Flame (n.d.) EduBeans. Retrieved from https://www.edubeans.com/Class_VIII_Science_Combustion-and-Flame.php.
Helmenstine, A.M. (2020). Types of Chemical Reactions. ThoughtCo. Retrieved from https://www.thoughtco.com/types-of-chemical-reactions-604038.
Types of Chemical Reactions. (2019, June 5). LibreTexts. Retrieved from https://chem.libretexts.org/@go/page/79224