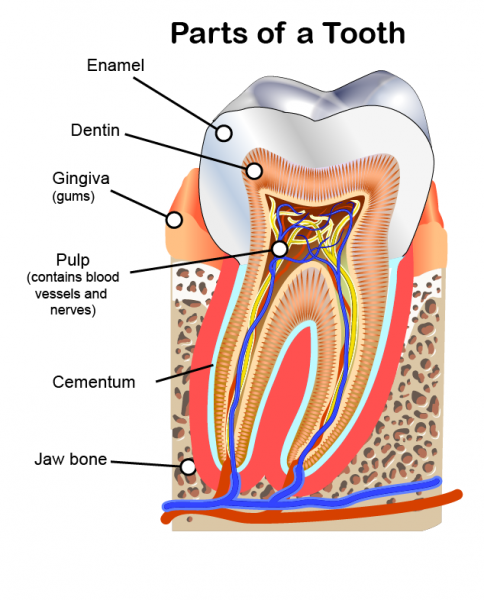
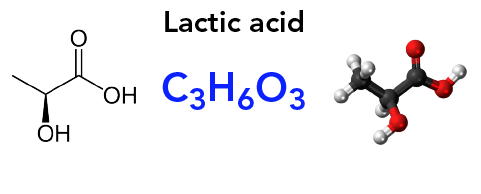
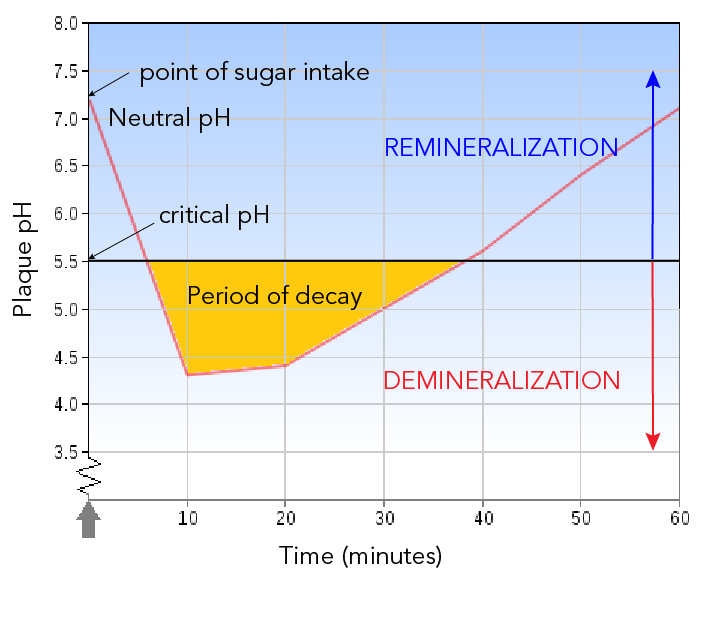
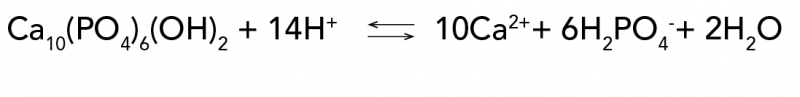How can chemistry help you take care of your teeth?

Cartoon of people cleaning teeth (IconicBestiary, iStockphoto)

Cartoon of people cleaning teeth (IconicBestiary, iStockphoto)
7.49
How does this align with my curriculum?
Curriculum Alignment
BC
12
Chemistry 12 (June 2018)
Big Idea: Dynamic equilibrium can be shifted by changes to the surrounding conditions.
YT
12
Chemistry 12 (British Columbia, June 2018)
Big Idea: Dynamic equilibrium can be shifted by changes to the surrounding conditions.
NU
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit C: Disease Defence and Human Health
YT
8
Science Grade 8 (British Columbia, June 2016)
Big Idea: Life processes are performed at the cellular level.
NT
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit C: Disease Defence and Human Health
AB
11
Knowledge and Employability Science 20-4 (2006)
Unit A: Applications of Matter and Chemical Change
BC
10
Science Grade 10 (March 2018)
Big Idea: Energy change is required as atoms rearrange in chemical processes.
BC
12
Chemistry 12 (June 2018)
Big Idea: Acid or base strength depends on the degree of ion dissociation.
NU
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit A: Applications of Matter and Chemical Change
NU
12
Chemistry 30 (Alberta, 2007, Updated 2014)
Unit D: Chemical Equilibrium Focusing on Acid-Base Systems
NU
10
Science Grade 10 (British Columbia, June 2016)
Big Idea: Energy change is required as atoms rearrange in chemical processes.
ON
10
Science Grade 10 Applied (SNC2P)
Strand C: Chemical Reactions and Their Practical Applications
YT
11
Chemistry 11 (British Columbia, June 2018)
Big Idea: Matter and energy are conserved in chemical reactions.
YT
12
Chemistry 12 (British Columbia, June 2018)
Big Idea: Acid or base strength depends on the degree of ion dissociation.
NT
11
Knowledge and Employability Science 20-4 (Alberta, 2006)
Unit A: Applications of Matter and Chemical Change