Carbon Dioxide: Outdoors and Indoors
CO2 symbol in front of a school (Let’s Talk Science)

CO2 symbol in front of a school (Let’s Talk Science)
How does this align with my curriculum?
Learn about the importance of carbon dioxide, its sources and how people control it indoors.
Why is carbon dioxide important to living things?
Carbon is found in all living things. In fact, life on Earth would not be possible without carbon. One reason is because carbon combines with other atoms to form important molecules. These include DNA, sugar and carbon dioxide (CO2).
Plants use energy from the Sun to combine carbon dioxide and water to form oxygen and glucose. We call this process photosynthesis.
Image - Text Version
Shown is a colour photograph of colourful vegetables. Vegetables fill the whole image. They include dark, leafy greens, red and orange tomatoes in various sizes, red, green and yellow peppers, heads of white cauliflower and a lemon.
Glucose is a type of sugar. Sugar molecules store energy from the Sun in the bonds that hold their atoms together.
Plants use some glucose for their life processes. They convert the rest of the glucose into molecules such as starch and protein. These molecules store much more energy than sugar.
Image - Text Version
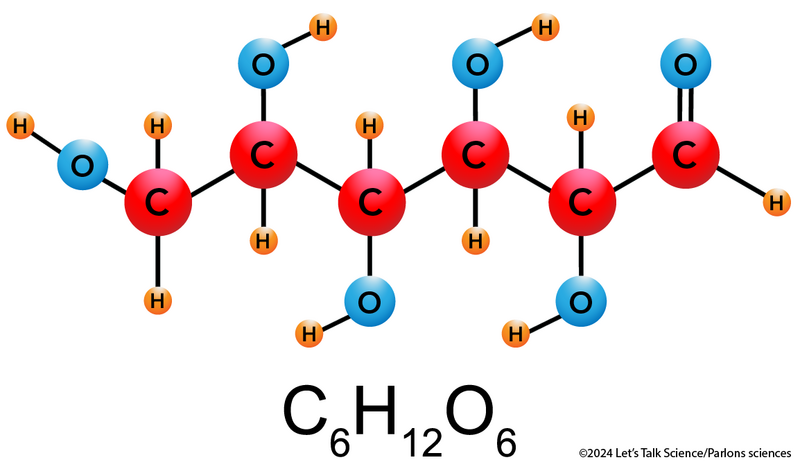
Shown is a colour ball and stick diagram of a glucose molecule. There are 6 red, 6 blue, and 12 orange balls connected by black lines, The largest spheres are red and labelled “C.” These represent carbon atoms. The medium-sized blue spheres are labelled “O.” These represent oxygen atoms. The small orange spheres are labelled “H.” These represent hydrogen atoms. Below the drawing is the title C subscript 6 H subscript 12 and O subscript 6. The six carbon atoms are connected by single black lines in a horizontal zigzag pattern. Five of the oxygen atoms are connected to the carbon by single lines. One is connected with a double line. Eight hydrogen atoms are connected to the carbon atoms by single lines. The other four hydrogen atoms are connected to oxygen atoms by single lines.
When plants and animals need energy, the ribosomes in their cells spring into action. Ribosomes combine oxygen with glucose. This releases the energy stored in the bonds. We call this process cellular respiration. As part of this process, water and CO2 are produced. They are then released into the atmosphere.
Did you know?
We release CO2 into the air every time we breathe. That is about 22 000 times per day!
Carbon Dioxide Outdoors
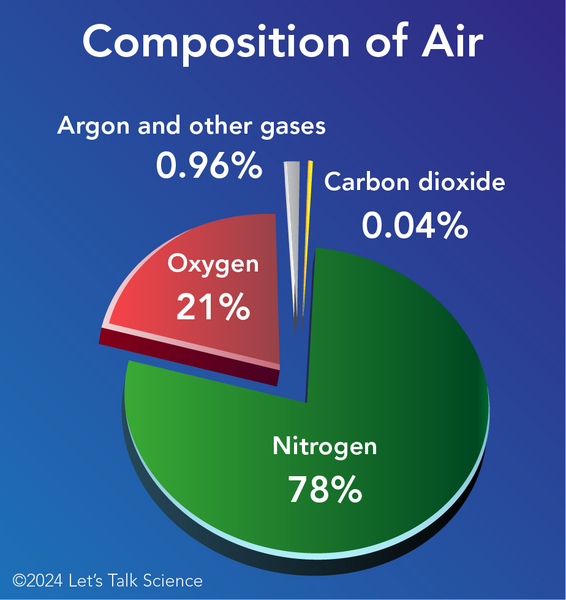
Air is about 0.04% of CO2. The rest is nitrogen (78%), oxygen (21%), and small amounts of other gases.
Did you know?
Before the mid-1700s, the concentration of CO2 in Earth’s atmosphere was less than 280 parts per million (ppm). As of May 1, 2024 it was up to 424.45 ppm.
Image - Text Version
Shown is a colour pie graph showing the composition of the gases found in the atmosphere. The title, “Composition of Air” is in white across the top of the illustration. The pie is thick and flat, and its slices are separated from each other against a blue background. The nitrogen portion is shown in green. It is the largest section and it is labelled “nitrogen 78 percent”. The oxygen portion is shown in red. It is the second largest section and it is labelled “oxygen 21 percent”. A small grey portion is labelled “Argon and other gases 0.96 percent”. The smallest pink section is labelled “Carbon dioxide 0.04 percent”.
Carbon Sources and Sinks
We call something that releases carbon into the environment a “carbon source”. We call something that takes in more carbon than it releases a “carbon sink”. Some sources and sinks are natural. Others are the result of human activities. We call the cycling of carbon through land, water and air the carbon cycle. CO2 is one of the most important molecules in the carbon cycle.
Carbon Sources
Check out the interactive below to learn about natural and human-made sources of CO2.
Access a screen-reader friendly PDF
Carbon Sinks
Like carbon sources, carbon sinks can be natural or human-made.
Natural Carbon Sinks
Plants use CO2 for photosynthesis. This makes them an important natural carbon sink. Not only do plants store carbon when they are alive, they also store carbon after they die. This keeps the carbon from going into the atmosphere. Some habitats are very good carbon sinks. These include wetlands and forests.
Image - Text Version
Shown is a colour photograph of a wet marsh and a conifer forest. The lower half of the photograph is flat, rippling water. Long grass grows in patchy clumps. A handful of dead trees are standing at the edge of the water, and several thin, bare trunks lie across the surface. In the background is a forest thick with evergreen trees.
Did you know?
When fossil fuels are burned, they go from being carbon sinks to carbon sources.
Oceans are another important natural sink. Oceans absorb and store carbon in two ways.
As Chemicals in Water
CO2 from air can dissolve in water. Carbonic acid and bicarbonate ions are the products of this reaction. They keep carbon from going into the atmosphere.
In Living Things
Most aquatic plants use carbon dissolved in water for photosynthesis. This stores the carbon in the plants. The carbon moves through ocean food chains when plants are eaten by animals.
Image - Text Version
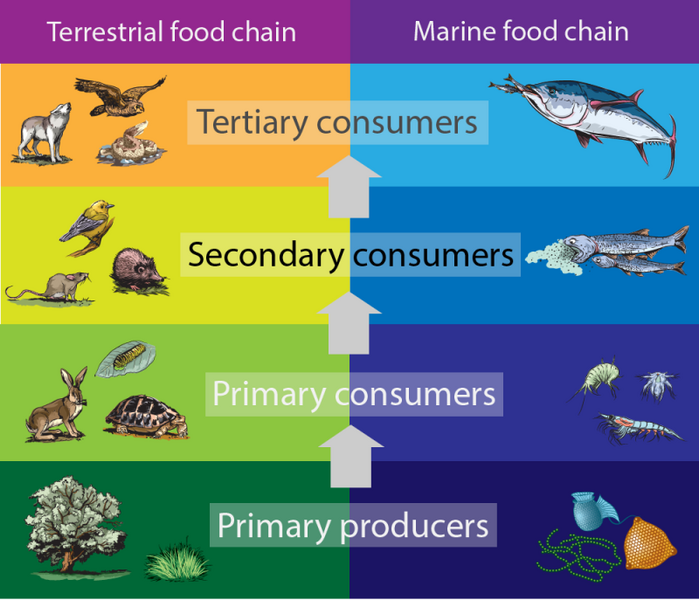
Shown is a colour, illustrated table showing food chains on land and in the ocean. The table has two columns. The left is titled “Terrestrial food chain.” The right is titled “Marine food chain.” Below are four rows with illustrations of different plants or animals on each side. Starting from the bottom, the first row is labelled “Primary producers.”The terrestrial side is dark green and has illustrations of a tree and grasses. The marine side is dark blue and has illustrations of bacteria and other microorganisms. In the middle, an arrow points to the row above. The second row up is labelled “Primary consumers.” The terrestrial side is light green and has illustrations of a rabbit, a turtle and an insect. The marine side is royal blue and has illustrations of krill, larvae and other zooplankton. The third row up is labelled “Secondary consumers.” The terrestrial side is yellow and has illustrations of a rat, a mole and a bird. The marine side is bright blue and has illustrations of long, silver fish eating clouds of something green and speckled. The fourth row up is labelled “Tertiary consumers.” The terrestrial side is orange and has illustrations of a coyote, a snake and a hawk. The marine side is light blue and has an illustration of a large, silver fish eating several smaller silver fish.
Did you know?
Human-made Carbon Sinks
People are also creating ways to capture and store CO2. This is often called Carbon capture and storage (CCS).
Image - Text Version
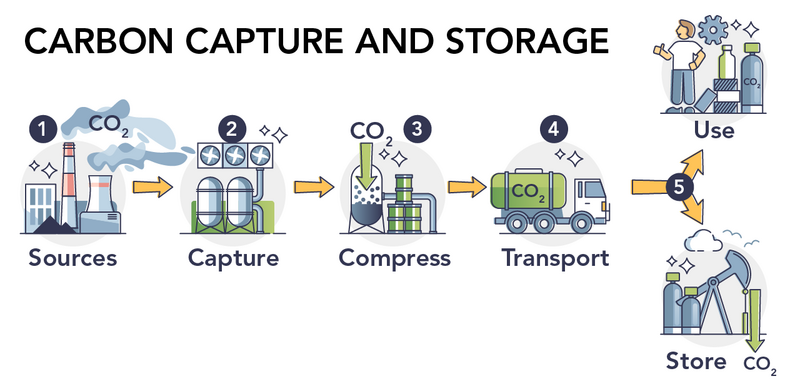
Shown is a colour illustration of carbon sources, capture, compression, transport, storage and use. Six numbered illustrations are connected by orange arrows, from left to right, on a white background. Illustration one is labelled “Sources.” It shows two smokestacks spewing grey gases labelled “CO subscript 2.” Illustration two is labelled “Capture.” It shows fans attached to two white tanks. Illustration three is labelled “Compress.” It shows blue, bubbling liquid inside one of the tanks. A green arrow labelled “CO subscript 2” points down from the top of the tank toward the surface of the liquid, near the bottom of the tank. Illustration four is labelled “Transport.” It shows a truck carrying a large green tank labelled “CO subscript 2.” The last orange arrow is divided so one tip points up and the other points down. This is labelled “5.” The top illustration is labelled “Use.” It shows a person standing next to several tanks and boxes, one of which is labelled “CO subscript 2..” A wheel above the person’s head indicates ideas or brainstorming. The bottom illustration is labelled “Store.” It shows tanks next to a pumpjack. A green arrow labelled “CO subscript 2” points into the ground below.
There are several steps in carbon capture and storage.
- Separate the CO2 from other gases.
- Trap or “capture” it.
- Compress the CO2 gas to form liquid CO2.
- Transport the liquid CO2 to where it will be stored or used.
- Store or use the CO2.
In Canada, carbon capture mainly happens at places that produce a lot of CO2. This includes electricity generation stations and in the oil and gas industry. CCS is not yet commonly used because it is very expensive.
De l’Islande au Québec, où en est le captage du carbone? [YouTube] Radio-Canada Info (6m 44s)
Carbon Dioxide and Climate Change
CO2 is a very important gas in the atmosphere. This is because it is a greenhouse gas. Greenhouse gases are often said to “trap” heat in the atmosphere. But how do they do this?
When energy from the Sun reaches Earth, much of it is absorbed by the land, water and air. As a result, these things radiate infrared energy. This energy travels right up to space unless something interacts with it.
Try this!
Hold your hand near dark pavement on a warm, sunny day. The heat you feel coming off it is caused by the movement of infrared energy.
Unlike oxygen and nitrogen molecules, CO2 molecules interact with infrared energy. The energy makes the molecules vibrate faster. The faster the molecules move, the higher their temperature. The vibrating CO2 molecules also bump into nearby molecules of oxygen and nitrogen. This makes them vibrate faster. This is what is causing the atmosphere to get warmer. Not only do the molecules move faster, they also radiate infrared energy in all directions. About half of the energy goes into space. The other half returns to Earth, causing the greenhouse effect.
CO2 experiment [YouTube] Uppsalainitiativet (1m 19s)
You might think that the Earth would be better off without greenhouse gases. But the Earth needs at least some of these gases to keep it at the right temperature. Not enough of these gases and the Earth would be too cold. Too much of these gases and the Earth would be too hot. Humans need to help keep these gases at a level that is just right.
Over the last 300 years, humans have been producing more CO2 than ever before. By adding more carbon sources and removing carbon sinks, we are causing the world's climate to get warmer. We call this warming trend climate change.
Carbon Dioxide Indoors
There are fewer sources and sinks of CO2 indoors than there are outdoors. One of the main sources is people. As people exhale, they release CO2 into the air. The more people there are in a room, the more CO2 there will be. Indoor animals and people smoking are also sources of CO2.
Image - Text Version
Shown is a colour photograph of students and a teacher posing in front of their classroom windows. Some students are sitting on the windowsills, others are sitting on top of nearby desks. The teacher stands on the left. They are all smiling and looking at the camera. Sunlight and green trees are visible through the window. More desks are arranged in a grid in front of them.
Also, some buildings are heated using fossil fuels such as wood, natural gas and oil. When these fuels are burned, they release CO2. This must be sent outdoors so that it does not build up indoors.
Image - Text Version
Shown is a colour photograph of orange flames around dark logs inside a fireplace. The flames are blue near the bottom where they reach up through what looks like glowing embers. Larger yellow and orange flames lick what looks like 3-4 whole logs. The box of the fireplace is lined in brick.
How do people control carbon dioxide indoors?
CO2 levels can increase in rooms and buildings with poor ventilation. Ventilation is the process of moving air into and within a room or building.
Natural ventilation involves the movement of air through openings such as doors and windows. Mechanical ventilation involves the movement of air using fans. Mechanical ventilation is often used with heating and cooling systems.
Did you know?
Many people have jobs in HVAC. This stands for heating, ventilation, and air conditioning.
Indoor ventilation can be improved by bringing in more outside air. This could be done by opening a window or a door. Clean air filters also help improve the air.
Image - Text Version
Shown is a colour photograph of a person in a hardhat studying a vent. The person is wearing bright yellow clothes and gloves with reflective strips. They are holding a tablet and looking closely at a grey metal box mounted high on a wall. On the ceiling above, wide metal tubes curve around wooden beams.
Information in this backgrounder is part of the Living Space Action Project.
Learn More
The Carbon Cycle
This lesson plan is part of the Let’s Talk Science Clothing4Climate project.
How do indoor environments impact physical and mental health? (2024)
This backgrounder by Let’s Talk Science describes how carbon dioxide, temperature and humidity affect people mentally and physically.
Carbon Dioxide and Space Missions (2024)
This backgrounder by Let’s Talk Science describes how people control carbon dioxide levels in spacecraft, such as the International Space Station.
The tricky plan to pull CO2 out of the air (2023)
This video explores some of the possibilities and challenges of carbon dioxide removal (CDR) systems.
Land - the planet’s carbon sink
This article, by the United Nations, explores the importance of land as a carbon sink.
But HOW Does Carbon Dioxide Trap Heat? (2023)
This video has an in depth explanation of the chemistry and physics behind how carbon dioxide “traps” heat. This video is best for high school students.
References
Canadian Centre for Occupational Health and Safety. (2019, June 6). Carbon dioxide.
Encyclopaedia Britannica. (2024, April 9). Carbon dioxide.
Fecht, S. (2021, February 25). How Exactly Does Carbon Dioxide Cause Global Warming? Columbia University.
Government of Canada. (2021, March 19). Carbon dioxide in your home.
Government of Canada. (2018, April 19). Factsheet: Ventilation and the indoor environment. Health Canada.
Government of Canada. (2021, April 23). Residential indoor air quality guidelines: Carbon dioxide. Health Canada.
International Atomic Energy Agency. (n.d.). The ocean carbon cycle.
Lindsey, R. (2024, April 9). Climate Change: Atmospheric Carbon Dioxide. Climate.gov.
MacLellan, A. (2016, April 5). Carbon dioxide levels at Mary Gardner school above 'acceptable level'. CBC.
Moseman, A. (2021, October 18). How are gases in the atmosphere analyzed and measured? MIT Climate Portal.
NASA. (2024, February). Carbon Dioxide | Vital Signs – Climate Change.