Introduction to Heat Transfer

Hands holding a hot mug (baza178, iStockphoto)

Hands holding a hot mug (baza178, iStockphoto)
How does this align with my curriculum?
Learn about the different ways that heat is transferred.
What is heat?
Think about all the ways that you can heat something up. You can boil water on the stove, rub your hands together quickly, or stand in front of a fire. But what is heat?
Heat is related to thermal energy. Thermal energy comes from the movement of tiny particles inside all matter. All solids, liquids, and gases are made up of small particles such as atoms and molecules. These particles have kinetic energy and are constantly moving. When these particles move more quickly, the amount of thermal energy increases.
Heat is thermal energy that is moving from one place to another. Heat flows from warmer objects to cooler objects. Since heat is a form of energy it is measured in Joules or sometimes in calories.
Misconception Alert
Objects don’t contain heat. They can contain thermal energy.
So what’s the difference between heat and temperature? Temperature tells us how hot or cold something is. Temperature is a measurement of an object’s average kinetic energy. Basically, it is a measure of the average motion of an object’s particles. Temperature is measured in degrees Celsius, degrees Fahrenheit, or using the Kelvin scale. Temperature and heat are connected. Heat is the flow of thermal energy between objects with different temperatures.
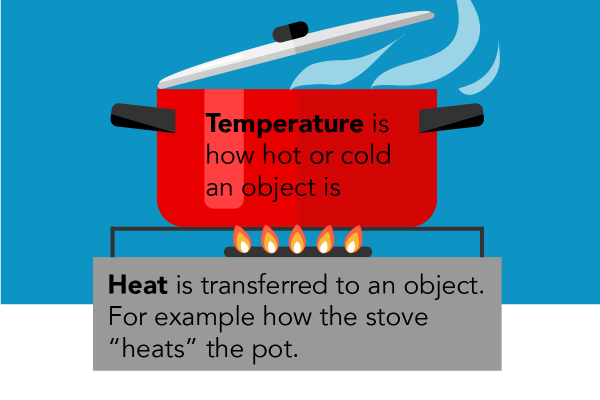
Image - Text Version
Shown is a colour illustration of a pot on a burner over flames. The pot is bright red. Its lid is open and steam is escaping. Across the front are the words, "Temperature is how hot or cold an object is." The pot sits on a black bracket over a burner with five flames. Below, a grey box contains the words, "Heat is transferred to an object. For example how the stove "heats" the pot.
Did you know?
A calorie is the amount of energy required to raise the temperature of 1 gram of water by 1 degree Celsius. The energy in the food you eat is measured in calories.
How is heat transferred?
Have you ever held a cup of hot chocolate after being outside in the cold? Holding a hot cup makes your hands feel warmer. What you are experiencing is the transfer of heat from one object to another. Heat energy from the hot chocolate is transferred to your hands.
When two objects have different temperatures, heat is transferred. The cooler object gets warmer until the two objects have the same temperature. Heat energy always flows from the warmer object to the cooler object.
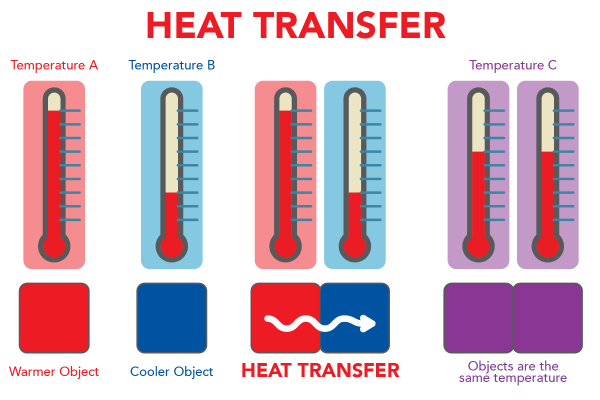
Image - Text Version
Shown is a colour diagram illustrating the process of heat transfer. The title, Heat Transfer, is in red block letters across the top. Below are three illustrations of the same pair of thermometers above a pair of coloured squares. The first illustration, on the left, shows the two squares quite far apart. The one on the left is red and labelled "Warmer Object." The first thermometer above, labelled "Temperature A," shows red liquid almost to the top. The square on the right is blue and labelled "Cooler Object." The thermometer above, labelled "Temperature B," shows red liquid just above the bottom of the scale. The second illustration shows the squares close together, touching sides. A squiggly white arrow points from the red square to the blue square. This is labelled "Heat Transfer." The thermometers above show the same temperatures as the first illustration. The third illustration shows both squares are now purple. This is labelled "Objects are the same temperature." Above, both thermometers show the same amount of red liquid. These are labelled "Temperature C."
Heat can be transferred in three ways:
- Conduction
- Convection
- Radiation
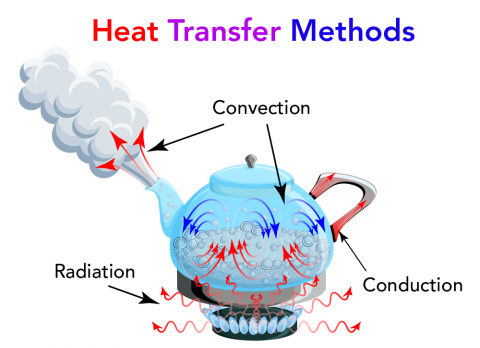
Image - Text Version
Shown is a colour illustration of three types of heat transfer happening inside a kettle on a stove. Starting at the bottom, blue flames rise from a round black burner. Red squiggly arrows extend above the flames in all directions. Some of these arrows reach through the bottom of the kettle. These are labelled "Radiation." Inside the kettle, the water is bubbling. Small red arrows curve from the bottom of the kettle up to the surface of the water. In the air above the water, small blue arrows curve down into the bubbles. Larger red arrows point out from the spout of the kettle. through steam. All these arrows are labelled "Convection." Another set of red arrows point from inside the kettle, along the handle. This glows red near the kettle sides. This is labelled "Conduction."
Conduction
Conduction happens when materials or objects are in direct contact with each other. The molecules in the warmer object vibrate faster than the ones in the cooler object. The faster vibrating molecules collide with the slower molecules. This makes the cooler molecules vibrate more quickly, and the object gets warmer. For example, have you ever sat on a cool couch? Did you notice how the seat was much warmer when you stood up? Heat from your skin was transferred to the couch through the vibration of molecules.
Conduction can also happen within a single object. Think of a metal rod that has just been poking around in a fireplace. The end of the rod that’s been touching the hot embers becomes very hot. Energy from the hot end will move through the rod to the colder end. Eventually, the temperature of the entire rod will be the same. This is why it is important to wear a glove when handling a hot metal rod!
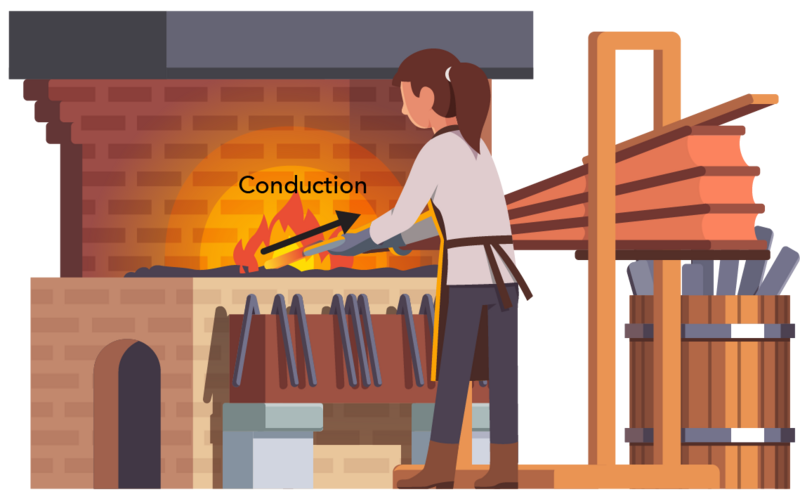
Image - Text Version
Shown is a colour illustration of a person holding a piece of metal in flames . An arrow pointing from the flames to the metal is labelled "Conduction." The person is shown from the back, wearing a long brown apron and grey gloves. The flames are on a surface about waist height. This is part of a large brick structure that looks similar to an oven. To the right is a large set of bellows and a barrel with more metal bars.
Some materials are better than others at conducting heat. You might have noticed this walking around your house in the winter. Have you ever noticed that your feet get much colder walking on bathroom tile than on carpet? This happens even though both the tile and the carpet are the same temperature as your house. However, tile is a much better conductor than carpet. More heat flows from your foot to the floor when walking on tile than carpet.
Thermal conductivity is a measure of how well a material conducts heat. Materials that are good at conducting heat are known as conductors. Metals, such as silver, copper, and aluminum are conductors. Materials that are not good at conducting heat and are known as insulators. Styrofoam, snow and fiberglass are examples of insulators. Many homes have insulation. Insulation keeps homes from losing too much heat energy to the surrounding air. Many common objects also provide insulation from air such as coolers, insulated flasks and sleeping bags.
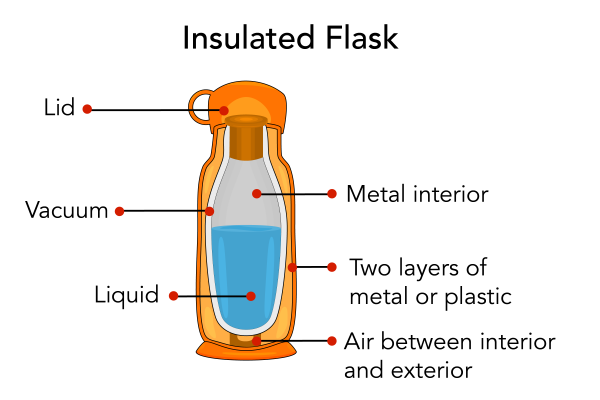
Image - Text Version
Shown is a colour illustration of the layers inside an insulated flask. The outside of the flask is orange and cylindrical. The inside is smaller and more rounded. The outside wall is labelled "Two layers of metal or plastic." The pale orange space inside that is labelled "Air between interior and exterior." The white space between the double walls of the inside container is labelled "Vacuum." The grey surface inside is labelled "Metal interior." A blue substance inside is labelled "Liquid." At the top is an orange cylinder with a large handle labelled "Lid."
Did you know?
Chefs like to use wooden spoons because wood is not a good conductor of heat. This means the spoons won't heat up too quickly and burn their hands.
Conduction usually happens in solids. The particles in liquids or gases are farther apart than in solids. This makes it easier for gas and liquid molecules to move around. Thus, liquids and gases more often transfer heat through convection.
Convection
Convection is another way that heat can be transferred. Convection is motion in a gas or liquid that is caused by temperature differences. This motion transfers heat throughout the gas and liquid. The molecules in liquids and gases are farther apart and have more room to move around than in solids. Because of this, heated liquid or gas molecules can physically move. This is different from conduction, where the molecules just vibrate more quickly.
Heating a pot of water on a burner is an example of convection. Heat transfers to water molecules at the bottom of the pot through conduction. These molecules start moving faster. The water at the bottom of the pot becomes less dense. It rises above the denser, cooler water. As the water rises, it carries heat energy upwards with it. Cooler water takes its place at the bottom of the pot where it is heated. This creates a circular cycle of heat transfer. This pattern is known as convection.
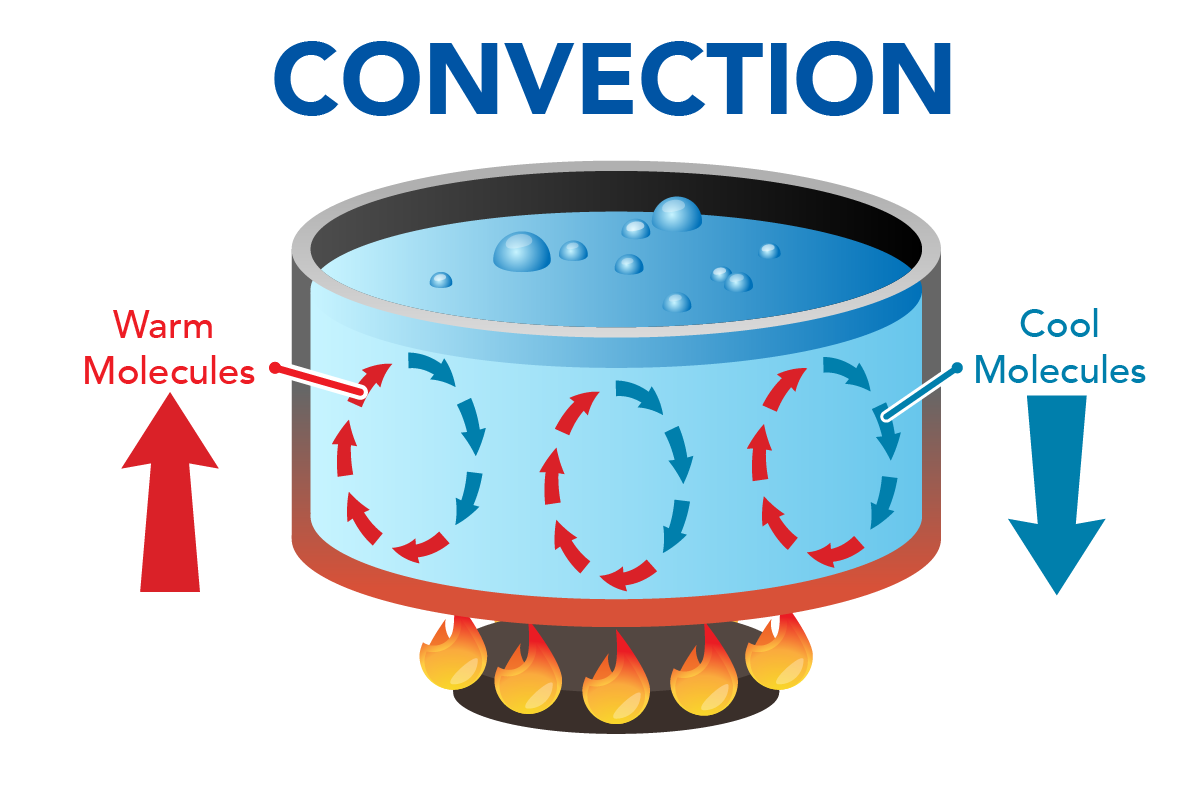
Image - Text Version
Shown is a colour diagram illustrating the motion of molecules inside a pot of boiling water. The title, "Convection" is in blue block letters across the top of the diagram. In the centre is a cross section of a large pot of pale blue, bubbling liquid. This sits above a round black object with flames around the edge. In the liquid are three vertical ovals of arrows moving clockwise between the surface of the water and the bottom of the pot. Red arrows move from the bottom up. These are labelled "Warm Molecules" with a large red arrow pointing up. Blue arrows move from the surface down. These are labelled "Cool Molecules" with a large blue arrow pointing down.
Convection plays a very important role in wind and ocean currents. For example, air over land is generally warmer than air over the ocean. The warmer air heats up and rises. It is then replaced by cooler air from above the ocean. We experience this movement of air as wind.
Radiation
Radiation is the third type of heat transfer. Unlike convection and conduction, no matter is needed for radiation. Thermal radiation is the transfer of energy via electromagnetic waves. Electromagnetic waves carry energy across space. Thermal radiation is the way that the Sun heats the Earth. The Sun’s energy travels in waves through space, not through atoms or molecules. Other warm objects, such as a toaster or your body, also radiate heat energy. A microwave also uses radiation to heat your food.
Image - Text Version
Shown is a colour illustration of radiation waves travelling from the Sun to the surface of the Earth. The Sun and Earth are shown against dark purple and black space with tiny, bright stars. The Sun is in the top left corner. It is a yellow sphere that glows with yellow and orange light. Earth is a bright blue sphere with the continents in bright green. Earth's atmosphere is illustrated with a thick layer of yellow, orange, red and purple around the planet. Long, yellow, wavy arrows stretch from the sun to the surface of the globe. They point to one side of the globe, illuminating the west coast of North America.
Heat Transfer in a House
An example of all three heat transfer processes occurring at the same time is the heating or cooling of a house.
- Conduction can either heat or cool the house. In the summer, heat is transferred from the warm air outside into the house through the walls or roof. In the winter, heat is transferred from the warm air inside the house out through the wall or roof.
- Convection occurs inside each room. Warmer air rises towards the ceiling and cooler air sinks towards the floor. Convection is also why the second floor of a house feels hotter than the basement.
- Thermal radiation from the Sun heats the roof of the house. Radiation can also transfer heat energy through windows.
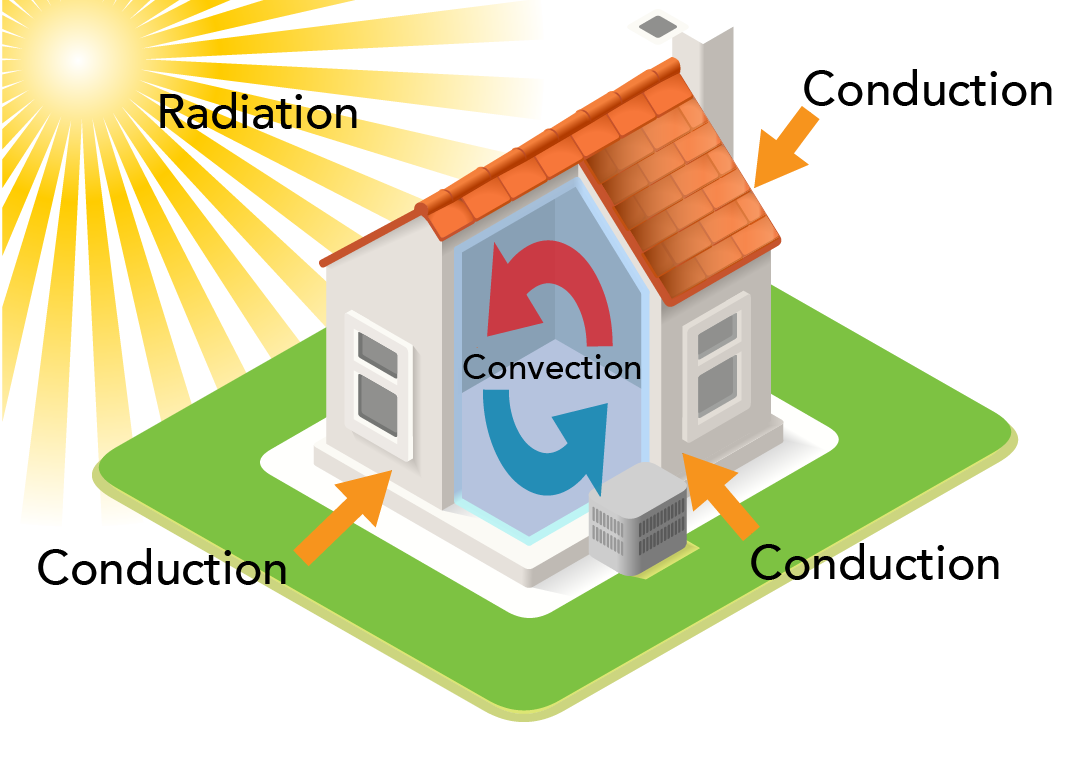
Image - Text Version
Shown is a colour diagram of a house with arrows indicating how heat is transferred in and around it. The house is small and square in the centre. It has a chimney, a red peaked roof and a strip of green grass around it. One corner is cut away to show the space inside. The sun shines in the top left corner. Its rays are long and yellow, reaching down to the house. This is labelled "Radiation." Three orange arrows point from outside the house to the walls and windows. These are labelled "Conduction." Inside the house, a red arrow curves up across the ceiling and down the wall. A blue arrow curves across the floor and up the wall. These are labelled "Convection.
We experience these different forms of heat transfer everyday. Understanding these concepts can lead to innovative uses of heat energy. For example, a Canadian teenager created a flashlight powered by the heat of your hand. Who knows what other ways we will use our knowledge of heat in the future.
Learn More
Thermal Imaging
Learn more about thermal imaging in this article by Let’s Talk Science.
Heat Transfer: Crash Course Engineering #14
This video (8:35 min.) from PBS explains heat transfer and the different mechanisms behind it.
Heat Capacity, Specific Heat, and Calorimetry
This video (4:13 min.) by Professor Dave explains how we can measure temperature changes.
There's No Such Thing As Cold (2015)
This video from It's Okay to Be Smart (5:01 min.) explains the difference between heat and temperature, why a wind makes us feel colder, and what it's like to live as a mass of jiggling atoms.
References
Campbell, A., Jenden, J., Lloyd, E., Tierney, M., Donev, M., (2017, August 29). Thermal Energy. University of Calgary Energy Education
NASA. (n.d.) Beat the Heat!
Perkins, S. (2016, September 30). Explainer: How heat moves. Science News for Students
University Corporation for Atmospheric Research, Centre for Science Education. (2018). Conduction.